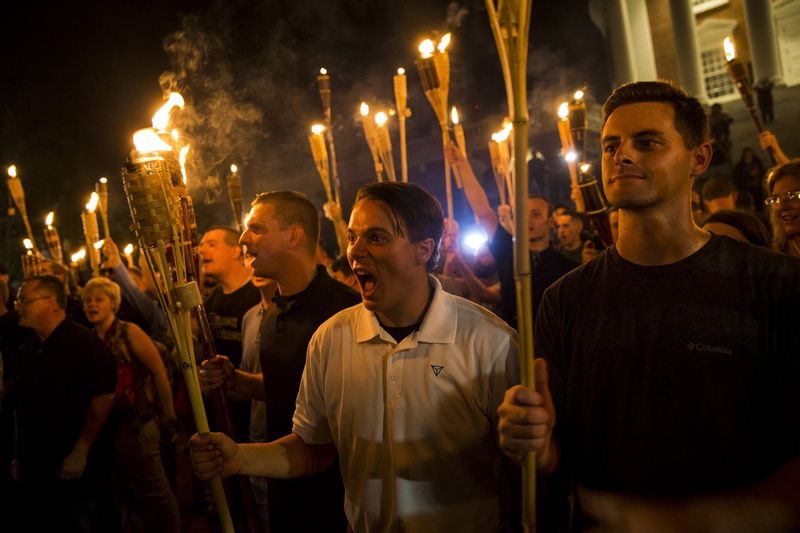BrownWatch

Get a Phfree sample edition of FUNKTIONARY





BOOKS BW IS CHECKING OUT






































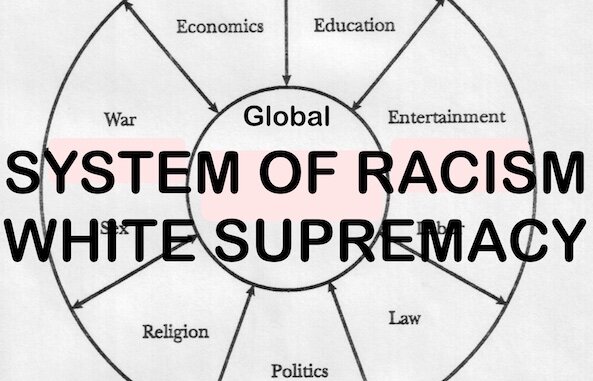
BrownWatch is a non-profit website that provides news and information for the purpose of liberation, education, self-reliance and greater self-knowledge. BrownWatch is intended to function as a tool to overcome info-gaps, verigaps and false programming toward the goal of “Endependence” within the meaning of FUNKTIONARY. It seeks to destroy the master/servant relationship maintaining the system of racism white supremacy and to unplug belief in systems of authority and all other granfalloons ensnaring humankind.
Any reproduction of copyrighted materials is done solely for the “productive uses” and “fair uses” of news reporting, sharing research, criticism, commentary, education and to provide an alternative contextual lens of such material within the meaning of the Copyright Act. 17 USCS § 107 (2019).
Powered by Squarespace.
Copyright © 2025, The BrownWatch. All rights reserved.