Father’s 20-Year Battle on Behalf of Vaccine-Injured Son Exposes Travesty of Liability-Free Vaccines
/From [CHD] In a riveting legal battle spanning two decades, William Yates Hazlehurst (“Yates”) on Feb. 2, 2022, became the first vaccine-injured person with a diagnosis of autism to reach a jury since the National Childhood Vaccine Injury Compensation Act of 1986 (the Vaccine Act) became law.
In a medical malpractice case filed in the Madison County Circuit Court in Tennessee, attorneys for Yates argued the clinic and physician who administered Yates’ vaccines, including the measles-mumps-rubella (MMR) vaccine on Feb. 8, 2001, should be held liable for medical malpractice and the neurological injuries Yates developed after being vaccinated.
Although the jury decided in favor of the physician — who Yates’ father said failed to adequately inform the parents of the risks of vaccinating Yates while he had an active ear infection — the case exposed major flaws in a system designed to protect children and shield pharmaceutical companies and physicians from liability for vaccine injuries.
“In the fight to end the autism epidemic, we were all hoping for the one knockout punch that would bring the truth to light and help end the autism epidemic,” Yates’ father, Rolf Hazlehurst, said.
“This medical malpractice trial was the only opportunity in the last 35 years for a jury to hear evidence in a court of law regarding whether a vaccine injury can cause neurological injury, including autism.”
Hazlehurst, who is a senior staff attorney for Children’s Health Defense (CHD), said “unless the Vaccine Act is repealed, my son is probably the only vaccine-injured child with a diagnosis of autism who will ever reach a jury.”
The Hazlehurst case was a medical malpractice case against the doctor who administered the pediatric vaccines that, in the opinion of the world’s top experts, sent Yates, now 22, spiraling into the depths of severe, non-verbal autism.
Although the case was originally filed in 2003, it didn’t receive its day in court for 19 years because a separate case involving Yates’ injury first had to work its way through the National Vaccine Injury Compensation Program (NVICP).
When Yates’ medical malpractice case was finally heard, the trial exposed alarming evidence about autism and vaccines, the low standard of care practiced by physicians administering pediatric vaccines and financial conflicts of interests between pharmaceutical companies that manufacture vaccines and government agencies entrusted with vaccine safety.
During the trial, the world’s top experts in the field of autism and mitochondrial disorderexplained how the administration of “routine” childhood immunizations can cause autism, brain injury, and many other disorders.
According to the National Institute of Mental Health, autism is a neurological and developmental disorder that affects how people interact with others, communicate, learn and behave. Symptoms can be severe and usually manifest before a child turns 3, which coincides with the age children receive the most childhood vaccines.
Increasing evidence indicates a significant proportion of individuals with autism have concurrent diseases such as mitochondrial dysfunction, abnormalities of energy generation, gastrointestinal abnormalities and abnormalities in the regulation of the immune system.
Yates’ medical malpractice trial illuminated how vaccines can cause autism in children with mitochondrial disorder and showed how the Vaccine Act — which is designed to ensure informed consent and compensation to injured children — is an abject failure because it’s largely unenforceable.
Yates was normal until he received his 12-month vaccines
During the first year of his life, Yates developed typically and met all of his developmental milestones.
“He was a happy, healthy and normal child,” his father said.
After his 6-month shots, Yates experienced a severe screaming episode approximately 24 hours after receiving the DTaP, Prevnar, Hib and Hep B vaccines.
In the days following his vaccinations, Yates began to experience seizure-like shaking episodes.
But his parents didn’t realize their son’s symptoms were consistent with a severe vaccine adverse reaction because they were not given a Vaccine Information Statement (VIS) at their pediatrician’s office.
According to the Centers for Disease Control and Prevention (CDC), a VIS is an information sheet produced by the CDC that explains both the benefits and risks of a vaccine to recipients.
“Federal law requires that healthcare staff provide a VIS to a patient, parent or legal representative before each dose of certain vaccines,” the CDC website states.
Instead of providing the VIS, Yates’ physician told his parents any adverse event to a vaccine would be “almost immediate” — within 5 to 15 minutes after vaccination.
Before Yates’ first birthday, his mother and aunt took him to the doctor because he had been sick, and his parents wanted to make sure it was okay for Yates to have a birthday party.
Hazlehurst told The Defender this appointment was not a scheduled well-child check. It was a sick visit. At the appointment, Yates was diagnosed with an ear infection and prescribed an antibiotic.
As the pediatrician turned to leave, he stated Yates would receive his shots, as it was close to his first birthday. A woman returned to the room who portrayed herself to be a nurse, but Hazlehurst later found out was only a medical assistant.
Yates’ mother asked the “nurse” whether their son should receive his shots despite being sick and was told he should.
Once again, they were not given a VIS form informing them of the risks of vaccinating Yates while he had a fever and an active ear infection.
“By administering vaccines to a sick child, the doctor and his clinic could charge a “modified double bill” Hazlehurst said.
That day, on Feb. 8, 2001, Yates received the MMR, Prevnar, Hib and Hep B vaccines. Twelve days later, Hazlehurst said his son experienced a high fever, rash and vomiting consistent with a vaccine adverse reaction.
Hazlehurst called the clinic where his son received his vaccine and talked to the doctor on call who asked him which vaccines Yates received. Hazlehurst responded, “whatever you get when you’re a year old.”
Hazlehurst was told his son was having an adverse reaction to the antibiotic and the doctor wrote him a prescription for a different antibiotic and an anti-fungal medication.
Soon after, Yates began to lose the skills he once had and began developing abnormally. He lost his speech, started running wild, was constantly on the go and would knock things off the table.
“He was visually ‘stimming’ off the falling objects and running with his head down for the visual stimulation,” Hazlehurst said.
He explained:
“It was not like he got the shots and boom, the next day he was autistic. That’s not the way it happened. The mitochondria produce the energy to the connecting tissue in the cells in the brain, and if they don’t get enough energy for a short period of time (as short as 6 seconds), cellular death occurs.
“The brain keeps developing, but it cannot develop normally because the connecting cellular tissue has been damaged. That’s why it takes time to manifest. It’s like watching grass grow. It’s happening, but you don’t realize it’s happening.”
Yates’ condition worsened. He developed an obsession with spinning objects, became a picky eater, started hand-flapping and toe-walking, became unable to sleep and exhibited gastrointestinal and multiple other medical and neurodevelopmental issues, Hazlehurst said.
On June 3, 2002, Yates was diagnosed with autism spectrum disorder.
Hazlehurst searches for answers to his son’s autism
According to federal law, there are specific recording requirements for vaccine medical records, and healthcare providers must provide records to a parent upon request.
Hazlehurst, on June 21, 2002, requested a copy of his son’s original vaccine records so other physicians could evaluate, diagnose and treat Yates.
Hazlehurst had questions about the American Academy of Pediatrics’ standard of care and wanted to know why his son was vaccinated while he was sick with a fever.
In response to Hazlehurst’s request and questions about Yates’ care, the pediatrician rushed out of the room and called his attorney, Hazlehurst said.
The doctor and clinic denied Hazlehurst’s requests to review and receive copies of his son’s original vaccine records, forcing him to petition the court for Yates’ records.
The court granted the request, and the local sheriff’s department seized Yates’ medical records from the doctor’s clinic.
Hazlehurst quickly realized there were problems with his son’s vaccine record, which was on an unsigned consent form that had a billing code sticker placed over the language regarding the risks and benefits of vaccines and vaccine information materials.
Hazlehurst said he never received a VIS form and Yates had been vaccinated without informed consent.
Hazlehurst files claim with the NVICP for son’s vaccine injury
Hazlehurst, like many parents of vaccine-injured children, pursued a claim with the NVICP as federal law requires. The process took nine years — from 2002 to 2011.
In order to bring a case in a court of law, the parents of a vaccine-injured child must first file their case with the NVICP.
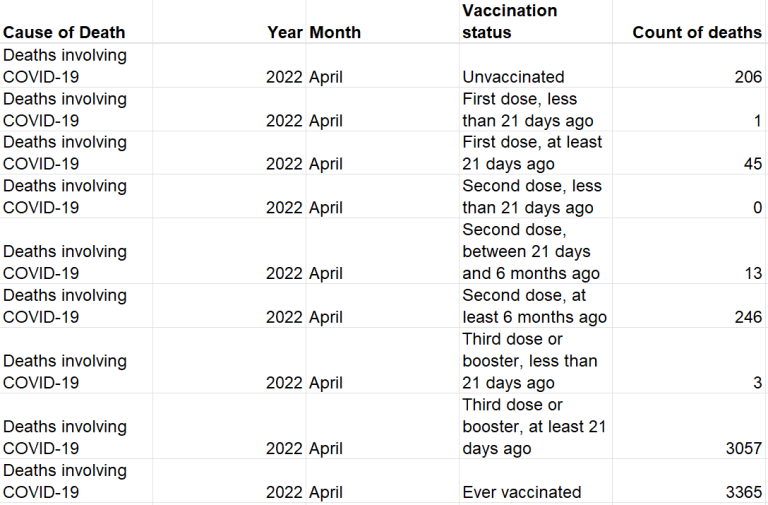
The NVICP is a special, no-fault tribunal housed within the U.S. Court of Federal Claims that handles injury claims for 16 federally recommended vaccines. To date, the court has awardedmore than $4 billion to thousands of people for vaccine injuries.
In the NVICP, America’s legal system is replaced by a “special master.” The special masters who review claims are government-appointed attorneys, many of whom are former U.S. Department of Justice (DOJ) attorneys.
Under the NVICP, the parents of vaccine-injured children are forced to sue the secretary of the U.S. Department of Health and Human Services (HHS) for compensation. HHS is represented by DOJ attorneys.
It is exceptionally difficult to obtain compensation within the NVICP, Hazlehurst said. The proceedings are often turned into drawn-out, contentious expert battles and the backlog of cases is substantial. Because of this, a single case can drag on for over a decade.
Payouts, including attorneys’ fees, are funded by a 75-cent tax per vaccine. There is a $250,000 cap on pain and suffering and death benefits.
The Vaccine Act established the NVICP, and the 2011 U.S. Supreme Court decision Bruesewitz et al v. Wyeth et al later guaranteed vaccine manufacturers, doctors and other vaccine administrators almost always have no legal accountability or financial liability in civil court when a government-recommended or mandated vaccine(s) causes permanent injury or death, Hazlehurst said.
The NVICP ultimately denied Yates’ claim, but his case against HHS became a central part of the U.S Supreme Court’s decision in Bruesewitz v. Wyeth.
Yates’ case in the NVICP was part of the Omnibus Autism Proceeding (OAP), in which 5,400 claims submitted to the NVICP were consolidated to determine if vaccines cause autism and if so, under what conditions.
“HHS whittled down the thousands of cases to six “test cases,” one of which was Yates’ case,” Hazlehurst said. “If HHS could find a way to deny NVICP compensation to the test cases, the agency would be able to deny compensation to all 5,400 families.”
Hazlehurst said HHS and the DOJ “took advantage of the fact that the rules of evidence, discovery and civil procedure mechanisms available in a regular court do not apply in the so-called vaccine court, and perpetrated fraud upon the special masters, the Court of Appeals for the D.C. Circuit and ultimately, the U.S. Supreme Court.”
The special masters on Feb. 12, 2009, in the so-called vaccine court, denied Yates’ petition for compensation and those of the five remaining OAP “test cases” involving children who developed autism after receiving their pediatric vaccines.
HHS makes key concession in Hannah Poling case
The potential fourth test case — Hannah Poling’s — was quietly conceded in 2007, in a corrupt coverup to conceal the opinion of the HHS expert witness, Dr. Andrew Zimmerman, the world’s leading expert in autism research, Hazlehurst said.
When Poling was 19 months old, she was vaccinated against nine diseases at one doctor’s visit: measles, mumps, rubella, polio, varicella, diphtheria, pertussis, tetanus and Haemophilus influenzae type b. In total, she received five vaccines.
Prior to receiving her vaccines, Poling was described as normal, happy, healthy, interactive, playful and communicative. But two days after being vaccinated, she was lethargic, irritable and febrile, and within 10 days she developed a rash consistent with vaccine-induced chicken pox.
Over the course of several months, Poling stopped eating, didn’t respond when spoken to, began showing signs of autism, developed neurological and psychological disorders and was diagnosed with encephalopathy caused by an underlying mitochondrial disorder.
In 2003, Poling’s father, Jon, a physician and trained neurologist, and mother, Terry, an attorney and nurse, filed an autism claim against HHS under the NVICP for their daughter’s injuries.
Five years later, the government settled the case before trial and in essence had it sealed.
During the OAP, in the Poling case, the government quietly conceded vaccines caused “regressive encephalopathy with features of autism spectrum disorder.”
According to CBS News, Poling received more than $1.5 million dollars for her life care, lost earnings and pain and suffering for the first year alone. After the first year, the family was supposed to receive more than $500,000 per year to pay for Poling’s care, which is estimated to amount to $40 million over her lifetime.
Jon Poling on March 6, 2008, said, “the results, in this case, may well signify a landmark decision with children developing autism following vaccinations.”
Prior to the Poling case, federal health agencies and professional organizations had reassured the public vaccines didn’t cause autism. The Poling case challenged that narrative, which is why the case was conceded and in essence sealed.
HHS’ concession that Poling developed autism as a result of a vaccine injury briefly became international news. Yet, only a handful of people knew why the government conceded Hannah’s case.
When news of the concession in Poling v. HHS was made public in March 2008, Dr. Julie Gerberding, then-director of the CDC, in an interview with CNN’s Dr. Sanjay Gupta said:
“We all know that vaccines can occasionally cause fevers in kids, so if a child was immunized, got a fever, had other complications from the vaccines, then if you are predisposed with a mitochondrial disorder, it can certainly set off some damage — some of the symptoms can be symptoms that have characteristics of autism.”
If HHS had not conceded her case, the truth as to how vaccines cause autism in some children with an underlying mitochondrial disorder would have been exposed by the world’s leading expert witnesses in the spotlight of the OAP, Hazlehurst said.
The concession document in the Poling case states:
“The vaccinations Hannah received on July 19, 2000, significantly aggravated an underlying mitochondrial disorder, which predisposed her to deficits in cellular energy metabolism, and manifested as a regressive encephalopathy with features of autism spectrum disorder.”
Zimmerman was an expert witness for the government defending vaccines in the NVICP. In 2007, during the hearing in the first test case, he told the government vaccines could cause autism in “exceptional” cases, but said the government later hid that information and misrepresented his expert opinion.
In a 2018 letter, Robert F. Kennedy, Jr., CHD chairman and chief legal counsel, and Hazlehurst meticulously described the DOJ’s fraud pertaining to the misrepresentation of Zimmerman’s opinions in the OAP and requested an investigation.
“The Office of Inspector General passed the buck to the DOJ Department of Ethics,” Hazlehurst said. “The DOJ investigated itself and wrote a highly misleading letter absolving itself of any wrongdoing.”
Zimmerman said in a signed affidavit:
“Shortly after I clarified my opinions with the DOJ attorneys, I was contacted by one of the junior DOJ attorneys and informed that I would no longer be needed as an expert witness on behalf of H.H.S. The telephone call … occurred after the above-referenced conversation on Friday, June 15, 2007, and before Monday, June 18, 2007. To the best of my recollection, I was scheduled to testify on behalf of H.H.S. on Monday, June 18, 2007.”
As a result of his firing, Zimmerman was not present for the Hazlehurst OAP proceedings, which allowed DOJ attorneys to misrepresent Zimmerman’s statements related to a separate autism case and apply them to all cases of autism, including Yates’ case.
Over the years Hazlehurst has repeatedly stated, “I want to be very clear, neither the Polings nor Dr. Zimmerman did anything wrong.”
“But,” he added, “if I did to a criminal, in a court of law, what the United States Department of Justice did to vaccine-injured children, I would be disbarred and I would be facing criminal charges.”
Zimmerman did testify as an expert witness on behalf of Yates in the medical malpractice case filed against Yates’ doctor, which was finally heard by a Tennessee court in February 2022.
Research by Zimmerman and others determined that at least 30%-40% of children with a diagnosis of regressive autism suffer from a mitochondrial disorder, which is a condition with which Yates was later diagnosed.
Yates in ‘perfect position’ to file lawsuit after exhausting remedies in NVICP
After exhausting all remedies under the NVICP — a process that took 25 years — the legal floodgates were then open, Hazlehurst said.
But because no one could sue the vaccine manufacturer, the only vaccine-injured child — out of thousands of cases originally included in the OAP — left with legal standing was Yates Hazlehurst and his claim of medical malpractice against the pediatrician who oversaw the administration of his vaccines.
Ultimately, the same medical experts, including Zimmerman and Dr. Richard Kelley, former director of the Genetics Department at Johns Hopkins Medical Institute — whose testimony HHS and the DOJ relied on in the Poling concession — concluded that what happened to Hannah Poling is what also happened to Yates Hazlehurst.
In an affidavit which was not admissible in the 2022 medical malpractice trial, Kelley stated:
“I also find, with a high degree of medical certainty, that the set of immunizations administered to Yates at 11 months while he was ill was the immediate cause of his autistic regression because of the effect of these immunizations to further impair the ability of his weakened mitochondria to supply adequate amounts of energy for the brain, the highest energy-consuming tissue in the body.”
Zimmerman’s expert opinion on the cause of Yates’ neurological condition was consistent with Kelley’s opinion. [MORE]