According to Leaked Documents U.S. and European Authorities Pressured Drug Regulators to Rush Approval of Pfizer-BioNTech’s COVID Vaccine Despite Safety Concerns
/From [CHD] U.S. and EU government officials pressured European drug regulators to rush approval of Pfizer-BioNTech’s COVID-19 vaccine despite safety concerns, according to leaked documents from the European Medicines Agency (EMA).
The EMA is the European equivalent of the U.S. Food and Drug Administration (FDA).
The documents, first reported on by Trial Site News, include emails, a PowerPoint presentation and a confidential Pfizer report from the Nov. 10-25, 2020, time period — just weeks before European, U.K. and U.S. regulators authorized the vaccine for emergency use.
Key revelations from the documents include:
A rush to quickly approve the vaccine, which was “pushed hard” by government figures in the U.S. and Europe.
Pressure on European regulators to approve the Pfizer vaccine despite experts’ concerns about the vaccine’s safety.
Significant differences in mRNA efficacy between vaccine trial batches and commercial batches of the Pfizer-BioNTech COVID-19 vaccine, raising safety concerns.
“No major interest” from the FDA regarding these discrepancies.
The lowering of the acceptable threshold of mRNA integrity, shortly before the Pfizer-BioNTech vaccine received regulatory approval in the U.K., U.S. and EU.
Direct lobbying by Pfizer CEO Albert Bourla to the president of the EU Commission and a high-level FDA regulator.
Political figures ‘pushed hard’ to ‘rush’ approval of Pfizer vaccine
A Nov. 16, 2020, email from Marco Cavaleri, then head of the EMA’s biological health threats and vaccines strategy, stated that “[Alex] Azar and US GOV [sic]” had “pushed hard” to “rush into EUA [Emergency Use Authorization].”
Azar at the time was secretary of the U.S. Department of Health and Human Services, which oversees the FDA.
In a Nov. 19, 2020, email, Noel Wathion, then deputy executive director of the EMA, referenced a “TC” — shorthand for teleconference — “with the commissioner,” referring to European Commissioner Ursula von der Leyen.
During the call, which Wathion described as “rather tense, at times even a bit unpleasant,” von der Leyen warned the EMA what might happen “if the expectations are not being met” to quickly issue a CMA [Conditional Marketing Authorization] for the Pfizer-BioNTech vaccine, “irrespective if such expectations are realistic or not.”
In the same email, Wathion wrote:
“The political fall-out seems to be too high, even if the ‘technical level’ … could defend such a delay in order to make the outcome of the scientific review as robust as possible. …
“Although we know that whatever we do (speeding up the process to align as much as possible with the ‘approval’ timing by FDA/MHRA [Medicines and Healthcare products Regulatory Agency] versus taking the time needed to have robust assurance in particular as regards CMC [Chemistry, Manufacturing and Controls guidelines] and safety) EMA will have a very big challenge addressing questions and criticism from various parties … in case of a delay of several weeks.”
The “various parties” Wathion referenced included the European Commission, the European Parliament, the media and the general public.
Wathion went on to argue that “CMC, responsibility and accountability are certainly elements to be considered in my view.”
In a later email, dated Nov. 22, 2020, Wathion further revealed the pressure the agency was facing to issue a CMA for the Pfizer-BioNTech vaccine, writing:
“The likelihood that FDA (and also MHRA) will issue an EUA before a CMA is granted is extremely high. So we have to prepare for this.”
However, Wathion expressed concerns in the same email that such preparation might come at the expense of a proper scientific assessment of the Pfizer vaccine.
“We are speeding up as much as possible but we also need to make sure that our scientific assessment is as robust as possible,” Wathion wrote.
Wathion also said, “the lay public and the media will not understand the nuance” between an EUA or CMA on the one hand, and full authorization on the other. “For them, an ‘authorization’ is an authorization.”
In fact, the media often refer to the Pfizer, Moderna and Johnson & Johnson COVID-19 vaccines as “approved” when in fact, in the U.S., they are being administered under EUA.
Wathion suggested it was necessary “to address this going from damage limitation to proactive expectation management,” in reference to the possibility that U.S. and U.K. regulators would issue an EUA before the EMA issued its own CMA.
Did concerns about integrity, consistency of vaccine batches lead to lowered standards?
Other leaked documents reveal discrepancies in the consistency of the Pfizer vaccine batches and other safety concerns.
A Nov. 10, 2020, email from Cavaleri revealed the FDA was, at that time, aware of “some issues on CMC to be sorted out,” and concerns that “CMC will end up being the difficult bit.”
In the same email, he said the FDA may grant its EUA by Christmas 2020, and inquired whether the EMA could grant its own CMA “at the same time.”
The “issues” Cavaleri referred to pertained to a significant discrepancy in the mRNA integrity between the clinical batches and the proposed commercial batches of the Pfizer-BioNTech vaccine.
In a Nov. 23, 2020, email Evdokia Korakianiti, a scientific administrator with the EMA, addressed those issues, writing:
“Issue: A significant difference in %RNA integrity/truncated species has been observed between the clinical batches (~78% mRNA integrity) based on which the Interim analysis was performed and the proposed commercial batches (~55%).
“The company claims that the efficacy of the drug product is dependent on the expression of the delivered RNA, which requires a sufficiently intact RNA molecule.”
This had as-yet-unspecified implications for the safety of the product, as Korakianiti later explained in the same message:
“The root cause for the lower %RNA integrity at [sic] commercial batches has not yet been identified.
“The potential implications of this RNA integrity loss in commercial batches compared to clinical ones in terms of both safety and efficacy are yet to be defined.”
A confidential 43-page Pfizer report, also part of the leaked documents, provided further insight into the significance of this discrepancy.
According to the report, Acuitas Therapeutics, the company that developed the lipid nanoparticle platform used by the Pfizer-BioNTech and Moderna COVID-19 vaccines, had set “a minimum threshold” of mRNA integrity of “approximately 70%.”
The report states:
“The efficacy of the product is dependent on expression of the delivered RNA, which requires a sufficiently intact RNA molecule.”
In a Nov. 24, 2020, reply to Korakianiti’s email, Veronika Jekerle, head of the EMA’s pharmacy quality office, described these concerns as part of “a number of major concerns [that] remain that impact the benefit/risk of the vaccine (efficacy/safety).”
Jekerle said, “these concerns are shared by most member states” of the EU.
However, Jekerle suggested “an approval by the end of the year could potentially be possible if these concerns + GMP [good manufacturing practice] will be resolved.”
In an apparent contradiction, and perhaps revealing a shifting stance on the part of the EMA, a Nov. 23, 2020, email by Cavaleri stated: “…the issue on the mRNA content [is] not perceived as major.”
The same email also strongly implied the FDA felt similarly, as Cavaleri wrote there was “no major interest from [the] FDA.”
A Nov. 25, 2020, email from Jekerle further confirmed the lack of interest on the part of several regulators, including the FDA, regarding the mRNA integrity issue.
Jekerle wrote:
“FDA and Health Canada [HC] indicated that the safety concerns associated with variable species of mRNA/protein are more of a theoretical concern…
“FDA/HC/EMA agreed that alignment on specifications %B mRNA integrity are key in order to avoid that one regions [sic] gets all the suboptimal material … specifications should be clinically qualified.”
The above passage appears to indicate that specific vaccine batches would be “suboptimal” as a result of this discrepancy in mRNA integrity.
Jekerle’s Nov. 25, 2020, email also revealed further potential safety concerns — namely, that the “applicant has shared with FDA and us/MHRA only today an issue with visible particles in the DP [drug product] (appears to be lipid nanoparticle components).”
In other words, Pfizer, the “applicant,” revealed the concerns to regulators only on Nov. 25, 2020 — shortly before U.S., U.K. and EU regulators granted Pfizer the emergency and conditional approvals.
For instance, the MHRA approved the Pfizer vaccine on Dec. 2, 2020.
Concerns about mRNA integrity discrepancies appear to have been overcome, not by altering the product under consideration, but by changing the acceptable RNA integrity specification.
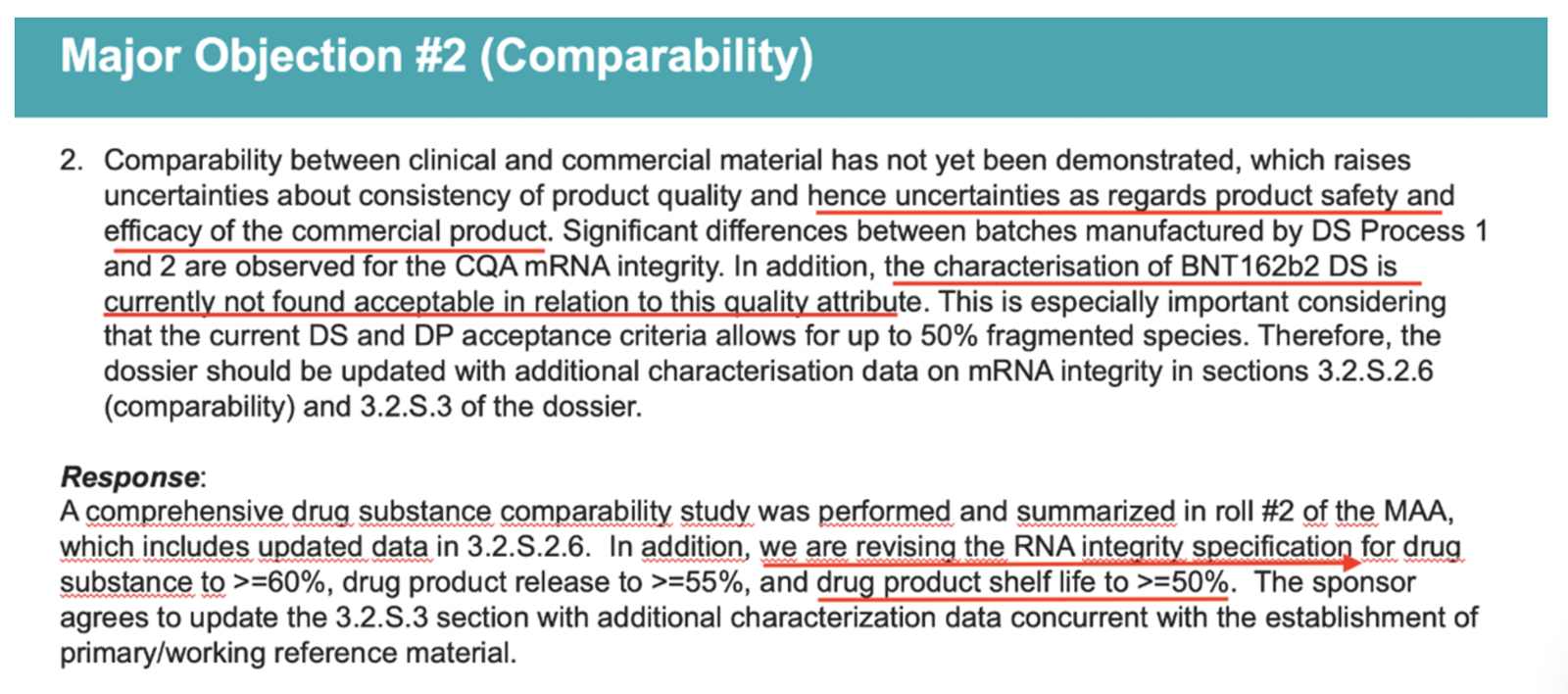
A leaked PowerPoint presentation that refers to a Nov. 26, 2020, meeting between the EMA and Pfizer, which took place just one day after Jekerle’s email, states:
“… we [the EMA] are revising the RNA integrity specification for drug substance to >=60%, drug product release to >=55%, and drug product shelf life to >=50%.”
These changes were made despite a mention in the same slide of “uncertainties as regards product safety and efficacy of the commercial product.”
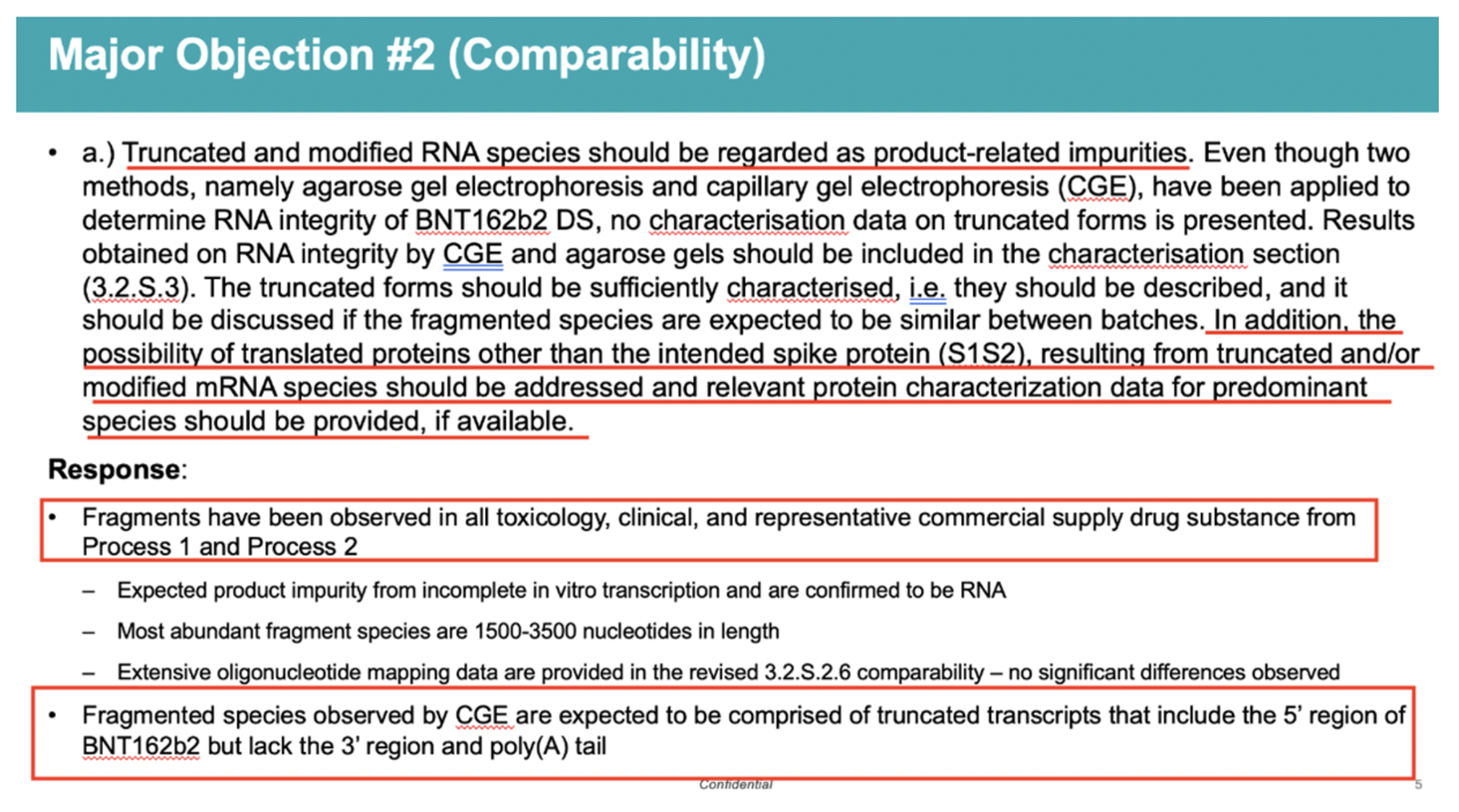
Another slide in the same presentation stated:
“Truncated and modified RNA species should be regarded as product-related impurities.
“In addition, the possibility of translated proteins other than the intended spike protein (S1S2), resulting from truncated and/or modified mRNA species should be addressed and relevant protein characterization data for predominant species should be provided, if available.”
Pharma execs lobby regulators for quick approval
The leaked documents revealed intense lobbying from high-level pharmaceutical and political figures in favor of a quick approval of the vaccine, despite the sentiment among EMA experts that a more robust scientific assessment of the Pfizer-BioNTech vaccine was needed.
For example, another email from Cavaleri explicitly mentions Pfizer’s direct lobbying of Dr. Peter Marks, the director of the FDA’s Center for Biologics Evaluation and Research.
Cavaleri wrote:
“Pfizer CEO lobbied Peter Marks telling him EMA wants the data earlier!!”
The same email mentioned that “colleagues” at the EMA “are pushing hard to compress [the] review timeframe” for Pfizer’s vaccine.
According to Trial Site News, such lobbying “could be interpreted as highly controversial.”
“Pfizer’s apparent access into the federal watchdog raises significant questions at the least,” Trial Site News reported, and “introduces the possibility for disturbing entanglements between industry and a purportedly independent, scientific federal agency.”
Trial Site News also referred to calls, in February 2022, on the part of independent members of the European Parliament for von der Leyen to resign, following revelations she had exchanged private text messages with Bourla.
While “only a small portion of these texts were ever disclosed,” Trial Site News said, the ones that were released “revealed her negotiating portions of a European-wide vaccine deal, unilaterally with Bourla, via a series of texts!”
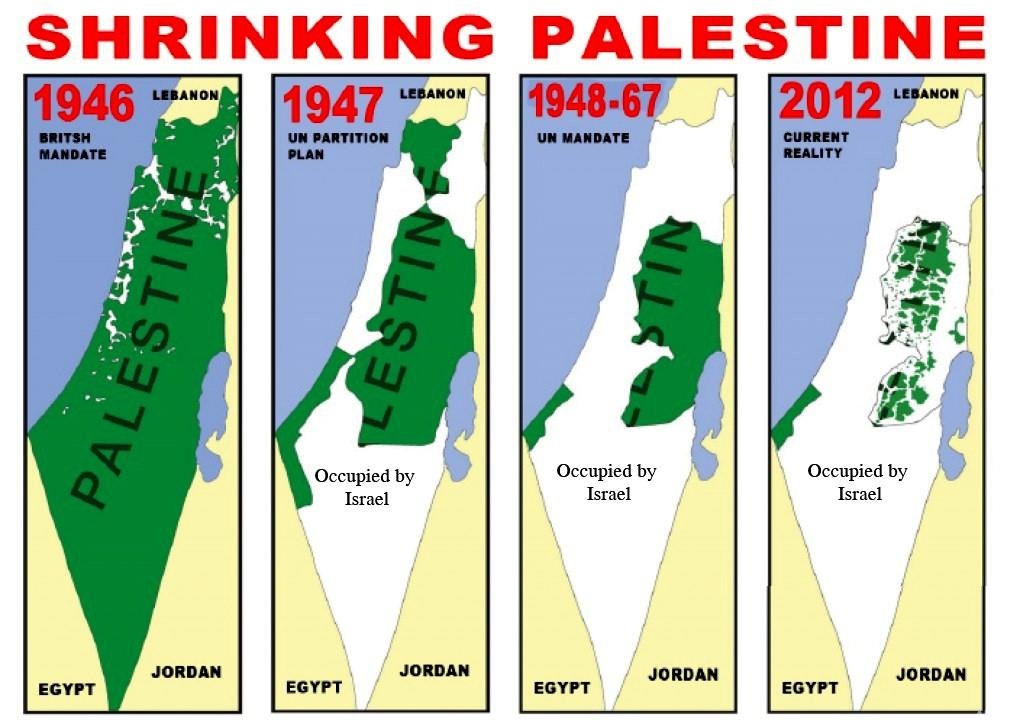
'Do You Believe the Expert Liars or Know From Seeing It with Your Own Eyes? Israel is Suffering Worse COVID than Palestine Due to Higher Vaccination Rates; More COVID Injections Caused More Deaths'
/FUCK BELIEFS WHEN YOU CAN SEE AND KNOW.
From [JOEL SMALLEY] The undeceiver and expert statistician explains,
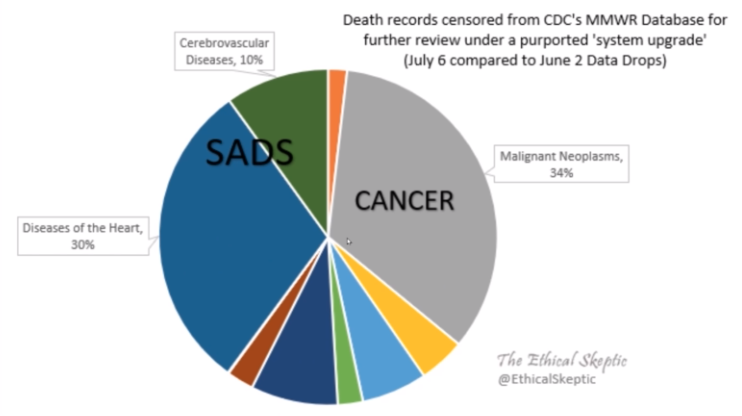
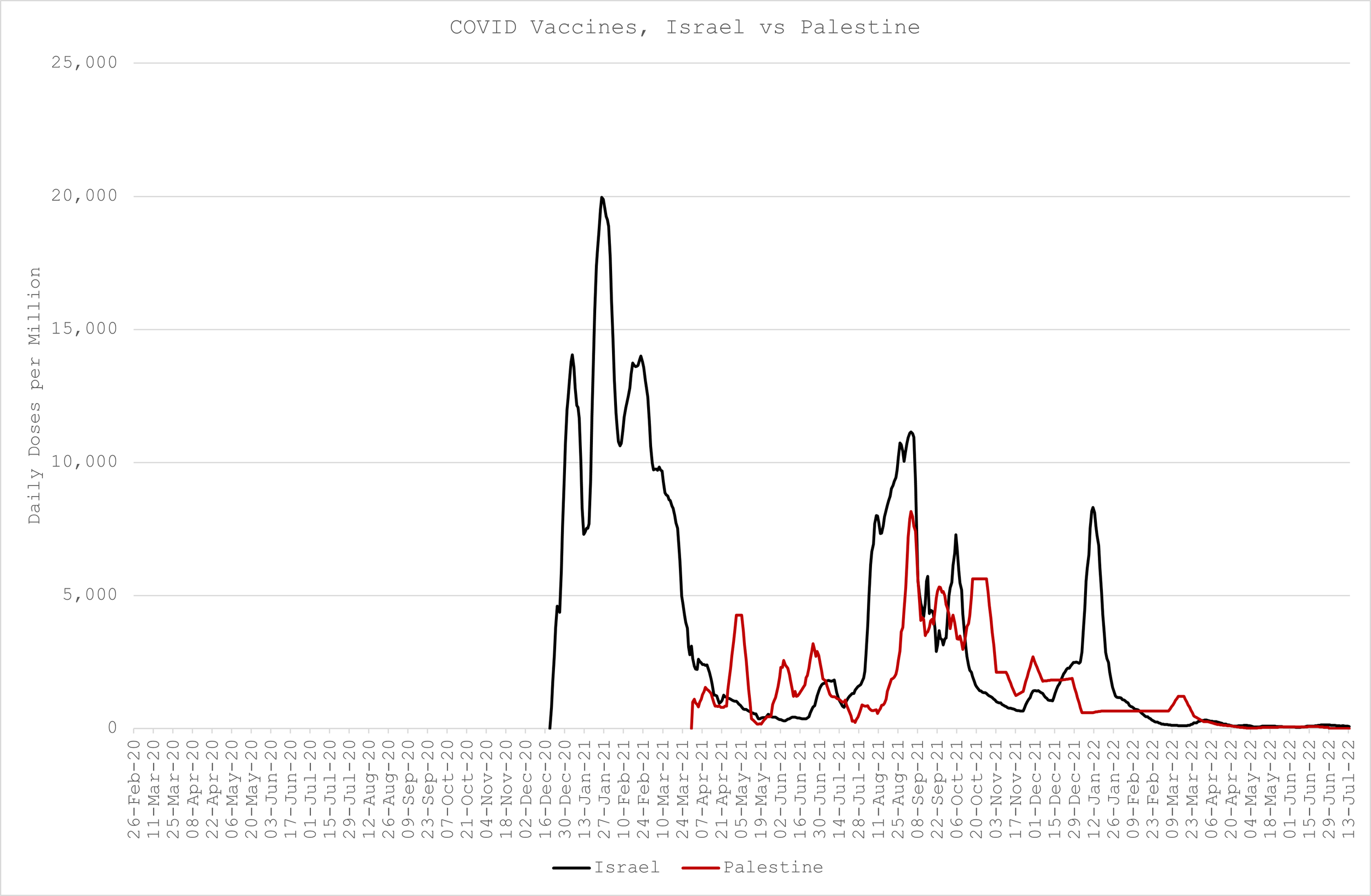
COVID Deaths
The magnitude and distribution of COVID deaths is somewhat similar in both countries apart from a double peak in Palestine in March and April ‘21, and a substantially larger peak in Israel in Feb ‘22 (Figure 2)
COVID “Vaccines”
Israel has “vaccinated” much more than Palestine. Palestine didn’t inject anyone until April ‘21, coincidentally just before their second deaths peak. They vaccinated almost at the same rate as Israel between August and November ‘21 but since then appear to have gotten wise to the dangers while their neighbour suffered the consequences of not. (Figure 3)
The Results
If we plot Israel minus Palestine deaths and “vaccines”, what do we get? Certainly not a picture of Safe & Effective™, that’s for sure! (Figure 4)
In fact, what is quite apparent is that relatively higher COVID deaths in Israel can be explained by relatively higher “vaccinations”.
You can believe the expert liars and the official dogma. Or, you can believe your own eyes.
Haiti Didn't Participate in the Plandemic and Remains Unaffected; Poor Country w/11M People Didn't Socially Distance, Wear Masks and 1% Injected. Hospitals Aren't Full of COVID Patients, Only 837 Dead
/From [HERE] Data from the World Health Organization (WHO) showed only 837 people have died in Haiti since the pandemic began, with a vaccination rate of 1.4% of the 11.6 million population.
“In Haiti, from 3 January 2020 to 5:03 pm CEST, 7 July 2022, there have been 31,703 confirmed cases of COVID-19 with 837 deaths, reported to WHO. As of 24 June 2022, a total of 342,724 vaccine doses have been administered,” according to the data from WHO.
As of June 24, only 1.4% of the population was fully vaccinated. Haiti had a population of 11,681,526 people as of Thursday, July 7, 2022, according to the data from Worldometers.
In contrast to countries that vaccinated the majority of their populations, Haiti has survived the impacts of Covid-19.
Haiti did not vaccinate its citizens. Their current vaccination rate is 1.4% of the population.
Haiti has not been effected by covid while the countries who did vaccinate the majority of their populations are struggling, and telling their citizens they need repeated injections. pic.twitter.com/23cBJBKqb7
— Frank Grimes Jr. (@FrankGrimes_Jr) July 5, 2022
In December 2021 a reported observed, “The term physical distancing has completely disappeared from discourse in Haiti. In both public and private institutions, people no longer wear masks and containers for washing hands have disappeared.
As a result, people live in total oblivion of the existence of the disease. Only some commercial banks, supermarkets and stores continue to demand the use of masks, in a context in which even the government authorities speak very little about the disease.” [MORE]
the Miami Herald reported: In Haiti, they are acting like COVID-19 doesn’t exist. Mask-wearing is an exception and not the norm; bands are playing to sold-out crowds; and Kanaval, the three-day pre-Lenten debauchery-encouraging street party is back on for February. . .
Across the border in the neighboring Dominican Republic, with roughly the same population, the pandemic has killed almost ten times the number, 2,364. Jet off to Miami-Dade County, home to one of the larger Haitian communities in the United States, and the death toll is even higher: 4,002 in a population of 2.7 million.
Haitians rejected the COVID injections and have returned hundred of thousands of unused doses donated by the U.S. The U.S. donated 500,000 doses of the Moderna Inc vaccine to Haiti in July 2021 through COVAX -- an abbreviation for COVID-19 Vaccines Global Access. According to Haiti’s health ministry, fewer than 66,800 doses were administered and only 20,354 people in the Caribbean nation of 11.4 million are fully vaccinated. [MORE]
Report Shows Pfizer Ignored Deaths that Occurred During Its COVID Shot Clinical Trial, Failed to Fully Investigate in order to Provide a Misleading Picture of Safety
/From [HERE] and [MORE] Three participants in the Pfizer Covid vaccine trial died shortly after vaccination and their deaths were not fully investigated, it has been revealed.
The revelations come in a report from Pfizer released on July 1st by court order as part of the documents which the U.S. FDA relied on to grant emergency use authorisation for the Pfizer vaccine in December 2020. They add to worries that adverse effects of the vaccine in the clinical trials were not properly documented, giving a potentially misleading picture of the drug's safety.
One of the deceased participants, a 56-year-old woman known as subject #10071101, was given two doses of the vaccine on July 30th and August 20th 2020 and died from a cardiac arrest two months later. In Pfizer's report on the participant it says:
In the opinion of the investigator, there was no reasonable possibility that the cardiac arrest was related to the study intervention of clinical trial procedures, as the death occurred two months after receiving Dose 2. Pfizer concurred with the investigator's causality assessment.
However, it's not clear how the investigator and Pfizer can be so sure the death was unrelated to the vaccine when there was no autopsy and no thorough medical assessment. As Sonia Elijah, who has analysed the report and summarised parts of it for Trial Site News, comments:
The conclusion that "there was no reasonable possibility" the vaccine could have caused the fatal cardiac arrest because "death occurred two months after receiving Dose 2" is not only presumptuous but also lacks a robust medical assessment. This is evident by the further comment that "it was unknown if an autopsy was performed". Why was there no follow up or inquiry into whether an autopsy was performed?
A second deceased participant was a 60 year-old man known as subject #11621327, who died three days after his first dose of vaccine, given on September 10th 2020. The report says that the "probable cause of death was progression of atherosclerotic disease". However, the subject had no known history of the condition (though was reported as obese). While the investigator deemed there to be "no reasonable possibility" of a link with the vaccine, seeing that it happened three days after the injection and "autopsy results were not available at the time" of the report, again, it's hard to see what that conclusion is based on.
A third deceased participant was a 72 year-old man known as subject #11521497, who received the first vaccine dose on October 7th and 19 days later, on October 26th, was admitted to hospital because "he fainted in the middle of the night". Reported as a syncope (temporary loss of consciousness usually related to insufficient blood flow to the brain), 16 days later, on November 11th, he died. The investigator claimed there was "no reasonable possibility that the syncope was related to the study intervention" and Pfizer said the syncope was "most likely coincidental". But again, it's unclear what this assessment is based on as the cause of death was reported as "unknown" and neither the investigator nor Pfizer attempted to investigate.
There were also a number of serious but non-fatal adverse events among trial vaccine recipients, but oddly, in every case where the trial investigator deemed it to be possibly related to the vaccine, Pfizer "did not concur", according to Sonia Elijah.
For example, a 71-year-old woman known as subject #11421247 developed severe ventricular arrhythmias in the evening following her second dose on October 14th 2020. The trial investigator wrote that "there was reasonable possibility that the ventricular arrhythmia was related to the study intervention [vaccine]". However, Pfizer stated there was "not enough evidence to establish causal relationship". This was despite arrhythmia being one of the adverse events of special interest (AESI) listed by Pfizer in its analysis of post-authorisation adverse event reports. [MORE]
Former Big Pharma Executive says FDA Colluded with Moderna to Bypass Vaccine Safety Standards. 'FDA Whitewashed Serious Signs of Health Damage and Lied to the Public on Behalf of the Manufacturers'
/From [CHD] According to an ex-pharmaceutical industry and biotech executive, documents obtained from the U.S. Department of Health and Human Services (HHS) on Moderna’s COVID-19 vaccine suggest the U.S. Food and Drug Administration (FDA) and Moderna colluded to bypass regulatory and scientific standards used to ensure products are safe.
Alexandra Latypova has spent 25 years in pharmaceutical research and development working with more than 60 companies worldwide to submit data to the FDA on hundreds of clinical trials.
After analyzing 699 pages of studies and test results “supposedly used by the FDA to clear Moderna’s mRNA platform-based mRNA-1273, or Spikevax,” Latypova told The Defender she believes U.S. health agencies are lying to the public on behalf of vaccine manufacturers.
“It is evident that the FDA and NIH [National Institutes of Health] colluded with Moderna to subvert the regulatory and scientific standards of drug safety testing,” Latypova said.
“They accepted fraudulent test designs, substitutions of test articles, glaring omissions and whitewashing of serious signs of health damage by the product, then lied to the public on behalf of the manufacturers.”
In an op-ed on Trial Site News, Latypova disclosed the following findings:
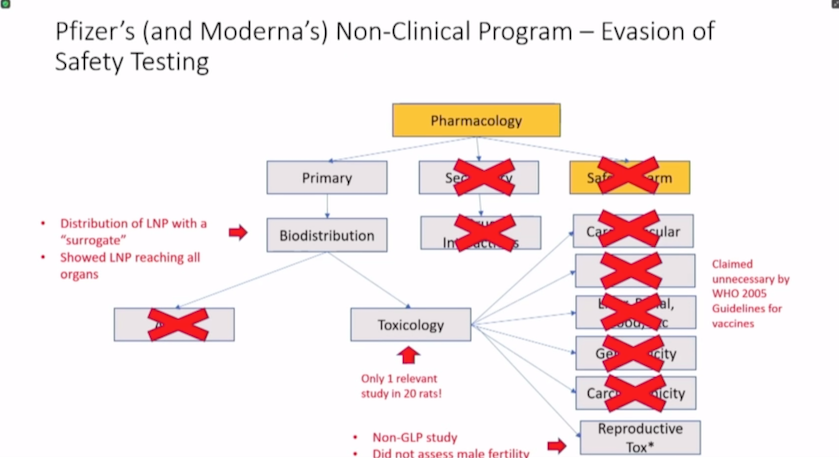
Moderna’s nonclinical summary contains mostly irrelevant materials.
Moderna claims the active substance — mRNA in Spikevax — does not need to be studied for toxicity and can be replaced with any other mRNA without further testing.
Moderna’s nonclinical program consisted of irrelevant studies of unapproved mRNAs and only one non-GLP [Good Laboratory Practice] toxicology study of mRNA-1273 — the active substance in Spikevax.
There are two separate investigational new drug numbers for mRNA-1273. One is held by Moderna, the other by the Division of Microbiology and Infectious Diseases within the NIH, representing a “serious conflict of interest.”
The FDA failed to question Moderna’s “scientifically dishonest studies” dismissing an “extremely significant risk” of vaccine-induced antibody-enhanced disease.
The FDA and Moderna lied about reproductive toxicology studies in public disclosures and product labeling.
“Moderna’s documents are poorly and often incompetently written — with numerous hypothetical statements unsupported by any data, proposed theories, and admission of using unvalidated assays and repetitive paragraphs throughout,” Latypova wrote.
“Quite shockingly, this represents the entire safety toxicology assessment for an extremely novel product that has gotten injected into millions of arms worldwide.”
Finding 1: Moderna’s non-clinical summary contains mostly irrelevant materials.
According to Latypova, about 80% of the materials disclosed by HHS that FDA considered in approving Moderna’s Spikevax pertain to other mRNA products unrelated to SARS-CoV-2 or COVID-19.
“Approximately 400 pages of the materials belong to a single biodistribution study in rats conducted at the Charles River facility in Canada for an irrelevant test article, mRNA-1674,” Latypova said. “This product is a construct of 6 different mRNAs studied for cytomegalovirus in 2017 and never approved for market.”
Latypova said the study showed lipid nanoparticles (LNPs) distribute throughout the entire body to all major organ systems.
Latypova found it odd the study protocol, report and amendments related to the study were copied numerous times throughout the HHS documents, suggesting Moderna may have been trying to meet a minimum word count.
In between the repetitive copies of the “same irrelevant study,” Latypova found “ModernaTX, Inc. 2.4 Nonclinical Overview” for Moderna’s COVID-19 vaccine with the investigational new drug application reference IND #19745.
Module 2.4, she said, is a standard part of the new drug application and is supposed to contain summaries of nonclinical studies.
“There are three separate versions of Module 2.4 included and many sections appear to be missing. It is not clear why multiple versions are included and there is no explanation provided as to which version specifically was used for the approval of Spikevax by the FDA.”
Latypova noted all three copies of Module 2.4 appear to have the same overview but reference a different set of statements and studies.
Latypova said the description of the finished supplied product differs between the two versions:
“Version 1 (p. 0001466) [says] mRNA-1273 is provided as a sterile liquid for injection at a concentration of 5 mg/mL in 20 mM trometamol (Tris) buffer containing 87 mg/mL sucrose and 10.7 mM sodium acetate, at pH 7.5.
“Version 2 (p. 0001499) [says] the mRNA-1273 Drug Product is provided as a sterile suspension for injection at a concentration of 20 mg/mL in 20 mM Tris buffer containing 87 g/L sucrose and 4.3 mM acetate, at pH 7.5.”
“It appears from reading section 2.4.1.2 Test Material (p.0001499) that Version 2 of the drug product had been used for manufacturing the Lot AMPDP-200005 which was used for nonclinical studies,” Latypova said. But “there is no explanation given for why the drug product in version 1 is different, and no comparability testing studies between the two product specifications are provided.”
Latypova pointed out that the package insert for FDA-approved Spikevax does not contain any information regarding the concentration of the product supplied in its vials.
Finding 2: Moderna said Spikevax mRNA does not need to be studied for toxicity and can be replaced with any other mRNA without further testing.
Latypova alleges Moderna, Pfizer and Janssen — manufacturer of the Johnson & Johnson shot — along with the FDA, have been deceptive in their assertions claiming the risks of COVID-19 vaccines are associated with the LNP delivery platform, and therefore, the mRNA “payload” does not need to undergo standard safety toxicological tests.
The documents state:
“The distribution, toxicity, and genotoxicity associated with mRNA vaccines formulated in LNPs are driven primarily by the composition of the LNPs and, to a lesser extent, by the biologic activity of the antigen(s) encoded by the mRNA. Therefore, the distribution study, Good Laboratory Practice (GLP)-compliant toxicology studies, and in vivo GLP-compliant genotoxicity study conducted with mRNA vaccines that encode various antigens developed with the Sponsor’s mRNA-based platform using SM 102-containing LNPs are considered supportive and BLA-enabling for mRNA-1273.”
Moderna is “claiming that the active drug substance of a novel medicine does not need to be tested for toxicity,” Latypova said. “This is analogous to claiming that a truck carrying food and a truck carrying explosives are the same thing. Ignore the cargo, focus on the vehicle.”
Latypova called the claim “preposterous,” as mRNAs and LNPs separately and together are “entirely novel chemical entities” that each require their own IND application and data dossier filed with regulators.
“Studies with one mRNA are no substitute for all others,” she added.
According to the European Medicines Agency, this chemical entity is entirely novel:
“The modified mRNA in the COVID-19 mRNA Vaccine is a chemical active substance that has not been previously authorized in medicinal products in the European Union. From a chemical structure point of view, the modified mRNA is not related to any other authorized substances. It is not structurally related as a salt, ester, ether, isomer, mixture of isomers, complex or derivative of an already approved active substance in the European Union.
“The modified mRNA is not an active metabolite of any active substance(s) approved in the European Union. The modified mRNA is not a pro-drug for any existing agent. The administration of the applied active substance does not expose patients to the same therapeutic moiety as already authorized active substance(s) in the European Union.
“A justification for these claims is provided in accordance with the ‘Reflection paper on the chemical structure and properties criteria to be considered for the evaluation of new active substance (NAS) status of chemical substances’ (EMA/CHMP/QWP/104223/2015), COVID-19 mRNA Vaccine is therefore classified as a New Active Substance and considered to be new in itself.”
“The reviewers specifically stated ‘modified RNA’ and not just the lipid envelope constitute the new chemical entity,” Latypova said. “All new chemical entities must undergo rigorous safety testing before they are approved as medicinal products in the United States, European Union and the rest of the world.”
Latypova said Moderna failed to cite any studies showing “all toxicity of the product resides with the lipid envelope and none with the payload” of the type and sequence of mRNA delivered to various tissues and organs.
“It is also not a matter of a mistake or rushing new technology to market under crisis conditions,” she added. “This scientifically fraudulent strategy was not only premeditated, it was also never really concealed.”
Latypova gave the example of a 2018 PowerPoint presentation by Moderna CEO Stéphane Bancel at a JP Morgan conference where he stated: “If mRNA works once, it will work many times.”
“This describes the deception practiced by the manufacturers, FDA, the Centers for Disease Control and Prevention (CDC), NIH and every government health authority or mainstream media talking head who participated in it,” Latypova said.
She continued:
“Imagine Ford Motor Company claiming that its crash testing program should be contained to the vehicle’s tires and that one test is sufficient for all vehicle models.
“After all both F150 and Taurus have tires, what’s in between the tires ‘worked once and will work again,’ and therefore it is inconsequential to safety, does not need to be separately tested and can be replaced at the manufacturer’s will with any new variation.
“This is the claim that Moderna, Pfizer, Janssen and other manufacturers of the gene therapy ‘platforms’ have utilized. Unlike Ford’s products, theirs have never worked as none of their mRNA-based gene therapy products have ever been approved for any indication. The fact that the regulators did not object to this argument raises an even greater alarm.”
“There is no question of incompetence or mistake,” Latypova said. “If this represents the current ‘gold standard’ of regulatory pharmaceutical science, I have very bad news regarding the safety of the entire supply or new medicines in the U.S. and the world.”
Finding 3: Moderna’s nonclinical program included only one non-GLP toxicology study of the active substance in Spikevax.
According to Latypova, a non-clinical program for a novel product usually includes information on pharmacology, pharmacokinetics, safety pharmacology, toxicology and other studies to determine the carcinogenicity or genotoxicity of a drug and its effects on reproduction.
The more novel the product, the more extensive the safety and toxicity evaluations need to be, she said.
In Module 2.4 described above, Latypova was able to identify 29 unique studies but only 10 were done with the correct mRNA-1273 test particle. The other studies were conducted using a “variety of unapproved experimental mRNAs unrelated to Spikevax or COVID illness.”
For example, the in-vivo genotoxicity studies included an irrelevant mRNA-1706 and a luciferasemRNA that is not in Moderna’s COVID-19 vaccine.
“Of the 10 studies using mRNA-1273, nine were pharmacology (‘efficacy’) studies and only one was a toxicology (‘safety’) study,” Latypova said. “All of these were non-GLP studies, i.e., research experiments conducted without validation standards acceptable for regulatory approval.”
There was only one toxicology study included in Moderna’s package related to the correct test particle mRNA-1273, but the study was non-GLP compliant, was conducted in rats and was not completed at the time the documents were submitted to the FDA for approval.
The results of the study were indicative of possible tissue damage, systemic inflammation and potential severe safety issues — and they are also dose-dependent, Latypova said. Moderna noted its findings but “simply moved on, deciding to forgo any further evaluation of these effects.”
Regarding reproductive toxicology, the only assessment was conducted on rats.
Pharmacokinetics — or the biodistribution, absorption, metabolism and excretion of a compound — were not studied with Moderna’s Spikevax mRNA-1273.
“Instead, Moderna included a set of studies with another, unrelated mRNA-1647 — a construct of six different mRNAs which was in development for cytomegalovirus in 2017 in a non-GLP compliant study,” Latypova said. “This product has not been approved for market and its current development status is unknown.”
Moderna claimed the LNP formulation of mRNA-1647 was the same as in Spikevax, so the study using this particle was “supportive of” the development of Spikevax.
“This claim is dishonest,” Latypova said. “While the kinetics of the product may be studied this way, the toxicities may not!”
“We do not know what happens with the organs and tissues when the delivered mRNA starts expressing spike proteins in those cells. This is a crucial safety-related issue, and both the manufacturer and the regulator were aware of it, yet chose to ignore it.
“The study demonstrated that the LNPs did not remain in the vaccination site exclusively, but were distributed in all organs analyzed, except the kidney. High concentrations were observed in lymph nodes and spleen and persisted in those organs at three days after the injection.
“The study was stopped before full clearance could be observed, therefore no knowledge exists on the full time-course of the biodistribution. Other organs where vaccine product was detected included bone marrow, brain, eye, heart, small intestine, liver, lung, stomach and testes.”
Given that LNPs of the mRNA-1647 were detected in these tissues, it’s reasonable to assume the same occurs with mRNA-1273 and “likewise would distribute in the same way,” Latypova said. “Therefore the spike protein would be expressed by the cells in those critical organ systems with unpredictable and possibly catastrophic effects.”
“Neither Moderna nor FDA wanted to evaluate this matter any further,” she added. “No metabolism, excretion, pharmacokinetic drug interactions or any other pharmacokinetic studies for mRNA-1273 were conducted,” nor were safety pharmacology assessments for any organ classes.
Finding 4: ‘Serious conflict of interest’ exists between Moderna and NIH.
According to Latypova, Moderna’s documents contain a letter from the Division of Microbiology and Infectious Diseases authorizing the FDA to refer to IND #19635 to support the review of Moderna’s own IND #19745 provided in “Module 1.4.”
Although Module 1.4 was not included in the documents provided by HHS, the FDA on Jan. 30 revealed the following timeline for Moderna’s Spikevax.
According to the FDA, Spikevax has two sponsors of its IND application package, including the NIH division that reports to Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases and chief medical advisor to President Biden.
The date of the pre-IND meeting for Spikevax was on Feb. 19, 2020. The IND submission for the NIH’s IND was on Feb. 20, 2020, while Moderna’s own IND was submitted on April 27, 2020.
According to the CDC, as of Jan. 11, 2020, Chinese health authorities had identified more than 40 human infections as part of the COVID-19 outbreak first reported on Dec. 31, 2020.
The World Health Organization on Jan. 9, 2020, announced the preliminary identification of the novel coronavirus. The record of Wuhan-Hu-1 includes sequence data, annotation and metadata from the virus isolated from a patient approximately two weeks prior.
Latypova said this raises several questions warranting further investigation:
Preparation for a pre-IND meeting is a process that typically takes several months, and is expensive and labor-consuming. How was it possible for the NIH and Moderna to have a pre-IND meeting for a Phase 1 human clinical trial scheduled with the FDA for a vaccine product a month before the COVID-19 pandemic was declared?
“How was it possible to have all materials prepared and the entire non-clinical testing process completed for this specific product related to a very specific virus which was only isolated and sequenced (so we were told) by Jan. 9, 2020?”
Ownership of the IND is both a legal and commercial matter, which in the case of a public-private partnership, must be transparently disclosed. “What is the precise commercial and legal arrangement between Moderna and NIH regarding Spikevax?”
“Does NIH financially benefit from sales of Moderna’s product? Who at NIH specifically?”
“Does forcing vaccination with the Moderna product via mandates, government-funded media campaigns and perverse government financial incentives to schools, healthcare system and employers represent a significant conflict of interest for the NIH as a financial beneficiary of these actions?”
“Does concealing important safety information by a financially interested party (NIH and Moderna) represent a conspiracy by the pharma-government cartel to defraud the public?”
Latypova further noted that immediately after the pre-IND meeting with the FDA, an “extremely heavy volume of orders for Moderna stock” began to be placed in the public markets.
This warrants an “additional investigation into the investors that were able to predict the spectacular future of the previously poorly performing stock with such timely precision,” she said.
Finding 5: FDA failed to question Moderna’s ‘scientifically dishonest studies’ dismissing an ‘extremely significant risk’ of vaccine-induced antibody-enhanced disease.
Moderna, prior to 2020, had never brought an approved drug to market.
“Its entire product development history was marked by numerous failures despite millions of dollars and lengthy time spent in development,” Latypova said. “Notably, its mRNA-based vaccines were associated with the antibody-dependent-enhancement phenomenon.”
For example, Moderna’s preclinical study of its mRNA-based Zika vaccine in mice showed all mice “uniformly [suffered from] lethal infection and severe disease due to antibody enhancement.”
The scientists were able to develop a type of vaccine that generated protection against Zika that “resulted in significantly less morbidity and mortality,” but all versions of the vaccine unequivocally led to some level of antibody-dependent-enhancement.
The Primary Pharmacology section for Spikevax includes nine studies evaluating immunogenicity, protection from viral replication and potential for vaccine-associated enhanced respiratory disease.
“These studies included the correct test article (mRNA-1273), however, all were non-GLP compliant,” Latypova said. The results of these studies are briefly summarized in the text of the document package, yet the study reports are not provided.
In the disclosed documents, Moderna claims “there were no established animal models” for SARS-CoV-2 virus due to its extreme novelty.
Yet, in the next sentence, “despite the extreme novelty of the virus,” Ralph Baric, Ph.D., at the University of North Carolina possessed an already mouse-adapted SARS-CoV-2 virus strain and provided it for some of Moderna’s studies, Latypova said.
According to Latypova’s assessment, there were other numerous contradictions in Moderna’s documents, and when enhanced disease risk was revealed in assays, the company waived off its own results with a statement regarding the invalidity of the assays and methods they used.
“As SARS-CoV-2 neutralization assays are, to this point, still highly variable and in the process of being further developed, optimized and validated, study measurements should not be considered a strong predictor of clinical outcomes, especially in the absence of results from a positive control that has demonstrated disease enhancement,” Moderna said.
“Clearly, both Moderna and FDA knew about disease enhancement and were aware of numerous examples of this dangerous phenomenon, including Moderna’s own Zika vaccine product of the same type,” Latypova said. “Yet, the FDA did not question Moderna’s scientifically dishonest ‘studies’ that dismissed this extremely significant risk without a proper study design.”
Finding 6: FDA and Moderna lied about reproductive toxicology studies in public disclosures and product labeling.
Although the FDA recommends Moderna’s COVID-19 vaccine for pregnant and lactating women, Moderna conducted only one reproductive toxicology study in pregnant and lactating rats using a human dose of 100 mcg of mRNA-1273.
Although the full study was excluded, a narrative summary of Moderna’s findings state, “high IgG antibodies to SARS-CoV-2 S-2P were also observed in GD 21 F1 fetuses and LD 21 F1 pups, indicating strong transfer of antibodies from dam to fetus and from dam to pup.”
Latypova said safety assessments in the study are very limited, but the following findings are described by Moderna:
“The mothers lost fur after vaccine administration, and it persisted for several days. No information on when it was fully resolved since the study was terminated before this could be assessed.”
In the rat pups, the following skeletal malformations were observed:
“In the F1 generation [rat pups], there were no mRNA-1273-related effects or changes in the following parameters: mortality, body weight, clinical observations, macroscopic observations, gross pathology, external or visceral malformations or variations, skeletal malformations, and mean number of ossification sites per fetus per litter.
“mRNA-1273-related variations in skeletal examination included statistically significant increases in the number of F1 rats with 1 or more wavy ribs and 1 or more rib nodules.
“Wavy ribs appeared in 6 fetuses and 4 litters with a fetal prevalence of 4.03% and a litter prevalence of 18.2%. Rib nodules appeared in 5 of those 6 fetuses.”
Moderna related the skeletal malformations to days when toxicity was observed in the mothers but waived away the finding as “unrelated to the vaccine,” Latypova said.
The FDA then “lied on Moderna’s behalf” in its Basis for Regulatory Action Summary document(p.14) stating “no skeletal malformations” occurred in the non-clinical study in rat pups despite the opposite reported by Moderna.
“No vaccine-related fetal malformations or variations and no adverse effect on postnatal development were observed in the study. Immunoglobulin G (IgG) responses to the pre-fusion stabilized spike protein antigen following immunization were observed in maternal samples and F1 generation rats indicating transfer of antibodies from mother to fetus and from mother to nursing pups.”
“In summary, the vaccine-derived antibodies transfer from mother to child,” Latypova said. “It was never assessed by Moderna whether the LNPs, mRNA and spike proteins transfer as well, but it is reasonable to assume that they do due to the mechanism of action of these products.”
Latypova said studies should have been done to assess the risks to the child by vaccinating pregnant or lactating women before recommending these groups receive a COVID-19 vaccine.
“We should ask the question why are they concealing the critical safety-related information from public, and making the product look better than the manufacturer has admitted,” Latypova said.
“The FDA did not have any objective scientific evidence excluding the skeletal malformations being related to the vaccine,” she added. “Thus, the information should have been disclosed fully in the label of this experimental and poorly tested product — not hidden from the public for over a year and then disclosed only under a court order.”
Latypova said FDA reviewers should have “easily seen through the blatant fraud, omissions, use of inadequate study designs and general lack of scientific rigor.”
The fact that more than half of the document package contains non-GLP studies for irrelevant, unapproved and previously failed chemical entities alone should have been sufficient reason to not approve this product, she added.
It would appear the FDA based its decision that the product is safe to administer to thousands of otherwise healthy humans on two studies in rats, Latypova said. The rest of the 700-page package was deemed to consist of “other supportive studies.”
The FDA noted studies were conducted in “five vaccines formulated in SM-102 lipid particles containing mRNAs encoding various viral glycoprotein antigens” but “failed to mention that these were five unapproved and previously failed products,” she said.
The regulators then concluded that using novel unapproved mRNAs in support of another unapproved novel mRNA was acceptable.
“The circular logic is astonishing,” Latypova said. Regulators allowed and personally promoted the use of failed experiments in support of a different and new experiment directly on the unsuspecting public.
Latypova called for the FDA, pharmaceutical manufacturers and “all other perpetrators of this fraud to be urgently stopped and investigated.”
David Martin, PhD., Presents Evidence that COVID Shots are Not Vaccines, but are an 'mRNA Spike Protein Instruction' that Tell the Body to Produce a Toxic Bioweapon, for Genocide and Depopulation
/From [MERCOLA] In this revealing interview with Greg Hunter of USAWatchdog.com, David Martin, Ph.D., presents evidence that COVID-19 injections are not vaccines but bioweapons that are being used as a form of genocide across the global population.1
In March 2022, Martin filed a federal lawsuit against President Biden, the Department of Health and Human Services and the Centers for Medicare and Medicaid Services alleging that COVID-19 shots turn the body into a biological weapons factory, manufacturing spike protein. Not only is the term "vaccination" misleading when referring to COVID-19 shots, it's inaccurate since they are actually a form of gene therapy.2
"And we are not only not going to be sued for, you know, any libel or misinformation, we are actually holding people criminally accountable for their domestic terrorism, their crimes against humanity and the story of the coronavirus weaponization that goes back to 1998," Martin says.3
SARS-CoV-2 Has Been in the Works for Decades
Martin has been in the business of tracking patent applications and approvals since 1998. His company, M-Cam International Innovation Risk Management, is the world's largest underwriter of intangible assets used in finance in 168 countries. M-Cam has also monitored biological and chemical weapons treaty violations on behalf of the U.S. government, following the anthrax scare in September 2001.4
According to Martin, there are more than 4,000 patents relating to the SARS coronavirus. His company has also done a comprehensive review of the financing of research involving the manipulation of coronaviruses that gave rise to SARS as a subclade of the beta coronavirus family.
Much of the research was funded by the National Institutes of Allergy and Infectious Diseases (NIAID) under the direction of Dr. Anthony Fauci.5 Martin explained:6
"I think it's important for your listeners and viewers to remember that it was 1999 when Anthony Fauci and Ralph Baric at the University of North Carolina Chapel Hill decided to start weaponizing coronavirus they patented in 2002 — and you heard that date correctly, that's a year before the SARS outbreak in China.
The first time they patented what they called an 'infectious replication defective chimera' of coronavirus. And let's unpack what that means.
Infectious means that it actually is more lethal to the target. Replication defective means its damage is primarily to the target and not to the target's family or friends or community or anything else. And in 2002, the University of North Carolina Chapel Hill patented the replication defective infectious coronavirus chimera, which then became the first instance of SARS.
And it was perfected in 2013 to 2016 during the gain of function moratorium, where the University of North Carolina Chapel Hill was given an exemption from the gain of function moratorium so they could continue to weaponize the virus to the point where in 2016, Ralph Baric published a paper in which he said the Wuhan Institute of Virology virus one, coronavirus, was 'poised for human emergence,' so they knew this all along.
You know, they knew it was a bioweapon since 2005. They knew it was effective at taking out populations, harming populations, intimidating and coercing populations. And they did that all very intentionally for the purpose of destroying humanity."
COVID-19 Shots Are an 'Act of Bioterrorism'
According to Martin, the spike protein that the COVID-19 shots manufacture is a computer simulation of a chimera of the spike protein of coronavirus. "It is, in fact, not a coronavirus vaccine. It is a spike protein instruction to make the human body produce a toxin, and that toxin has been scheduled as a known biologic agent of concern with respect to biological weapons for the last now decade and a half," he said.7
Rather than being a public health measure as they were widely campaigned to be, COVID-19 shots are an act of bioweapons and bioterrorism. Martin shared that in 2015, Dr. Peter Daszak, head of the EcoHealth Alliance that funneled research dollars from the NIAID to the Wuhan Institute of Virology for coronavirus research, stated:8
"We need to increase public understanding of the need for medical countermeasures such as a pan-coronavirus vaccine. A key driver is the media and the economics will follow the hype. We need to use that hype to our advantage, to get to the real issues. Investors will respond if they see profit at the end of the process."
Daszak, who Martin refers to as "the money launderer in chief," "actually stated that this entire exercise was a campaign of domestic terror to get the public to accept the universal vaccine platform using a known biological weapon. And that is their own words, not my interpretation," Martin said.9
Martin: 100 Million May Die Due to COVID Shots
Both Pfizer and Moderna's COVID-19 shots contain nucleic acid sequences that are not part of nature and have not been previously introduced to the human body. This amounts to a genetic engineering experiment that did not go through animal studies or clinical trials.
However, already people are dying from the shots and, Martin states, "many more will" due to issues such as blood clots, damage to the cardiovascular system and problems with liver, kidney and pulmonary function.10
An onslaught of reproductive and cancer cases related to the shots are also anticipated. "The fact of the matter is an enormous number of people who are injected are already carrying the seeds of their own demise," Martin said.11 As for how many may die, Martin believes the numbers may have been revealed back in 2011, when the World Health Organization announced their "decade of vaccination":12
"Based on their own 2011 estimate, and … this is a chilling estimate, but we just have to put it out there … When the Bill and Melinda Gates Foundation, the Chinese CDC, the Jeremy Farrar Wellcome Trust and others published The Decade of Vaccination for the World Health Organization back in 2011 their stated objective was a population reduction of 15% of the world's population.
Put that in perspective, that's about 700 million people dead … and that would put the U.S. participation in that certainly as a pro rata of injected population somewhere between 75 and 100 million people."
When asked what timeframe these people may die in, Martin suggested "there's a lot of economic reasons why people hope that it's between now and 2028."13 This is because of "a tiny little glitch on the horizon" — the projected illiquidity of the Social Security, Medicare and Medicaid programs by 2028.
"So the fewer people who are recipients of Social Security, Medicare and Medicaid, the better," Martin said. "Not surprisingly, it's probably one of the motivations that led to the recommendation that people over the age of 65 were the first ones getting injected."14 Other populations at risk are caregivers, including health care providers, and others in the workforce who were forced to be injected, such as pilots.
"Why is it that we're suddenly having 700 flights a day being canceled because, allegedly, airlines don't have pilots? … the dirty secret … is there a lot of pilots who are having microvascular problems and clotting problems, and that keeps them out of the cockpit, which is a good place to not have them if they're going to throw a clot for a stroke or a heart attack," Martin said.
"But the problem is we're going to start seeing that exact same phenomenon in the health care industry and at a much larger scale, which means we now have, in addition to the problem of the actual morbidity and mortality, meaning people getting sick and people dying.
We actually have that targeting the health care industry writ large, which means we are going to have doctors and nurses who are going to be among the sick and the dead. And that means that the sick and the dying also do not get care."15
Why COVID Shots May Change Your DNA
It's been stressed by the media and public health officials that COVID-19 shots do not alter DNA. However, Martin brings attention to a little-known grant from the National Science Foundation, known as Darwinian chemical systems,16 which involved research to incorporate mRNA into targeted genomes. According to Martin:17
"Moderna was started … on the back of a 10-year National Science Foundation grant. And that grant was called Darwinian chemical systems … the project that gave rise to the Moderna company itself was a project where they were specifically figuring out how to get mRNA to write itself into the genome of whatever target they were going after.
That could be a single-celled organism, it could be a multi-celled organism or it could be a human. And the fact of the matter is Moderna was started on the back of having proven that mRNA can be transfected and write itself into the human genome."
It is completely unknown what the short- or long-term effects of the spike protein analog that's inside people who received COVID-19 injections will be. But with respect to alteration of the genome, Martin states that data show mRNA has the capacity to write into the DNA of humans, and "as such, the long-term effects are not going to merely be symptomatic. The long-term effects are going to be the human genome of injected individuals is going to be altered."18
Fraud Removes Big Pharma's Liability Shield
The 2001 anthrax attack, which came out of medical and defense research, led to the passage of the PREP Act, which removed liability for manufacturers of emergency medical countermeasures.
This means that as long as the U.S. is under a state of emergency, things like COVID-19 "vaccines" are allowed under emergency use authorization. And as long as the emergency use authorization is in effect, the makers of these experimental gene therapies are not financially liable for any harm that comes from their use.
That is, provided they're "vaccines." If these injections are NOT vaccines, then the liability shield falls away, because there is no liability shield for a medical emergency countermeasure that is gene therapy. Further, lawsuits that can prove the companies engaged in fraud will also negate the liability shield. Martin states:19
"One of the convenient things about the PREP Act is the immunity shield from liability actually is only as good as the absence of fraud. Because if there was fraud in the promulgation of the events, leading to an emergency use authorization, then all of the immunity shield gets wiped out.
So the reason why it is so important for conversations like the one we're having to actually be promoted and be advanced is because the pharmaceutical companies — and this includes Pfizer and Moderna and J&J — know they are perpetuating a fraud. The great thing about this is when that fraud is established, 100% of the liability flows back to them.
… when a fraud was the basis for a fraud, then we actually have a number of other legal remedies that allow you to pierce that veil. So in the end, there's no question … and it's quite evident based on the current mortality and morbidity data that given the fact that when it comes to biological weapons and bioterror each count comes with $100 million penalty. That's what the federal statute gives us.
The penalty for corporate domestic terrorism, when you have per count $100 million a pop liabilities — that is an existential threat that takes a company like Pfizer or takes a company like Moderna out of existence. And that is what we're working for every day."
If you'd like to follow the progress of the ongoing legal cases seeking to expose the truth — that a criminal organization is seeking to obtain control over the global population via the creation of patented bioweapons marketed as novel viruses and injections — you can find all the details at ProsecuteNow.io, a website compiled by Martin and colleagues.20
- Sources and References
Authorities Force So-Called "COVID Positive” Citizens to Wear Ankle Bracelet Trackers to Monitor Their Movements in Hong Kong (a more secure free-range prison) Along with their "Cell Phones"
/From [HERE] The Wuhan coronavirus (COVID-19) apparently still exists in Hong Kong, where residents who are deemed to be “infected” are now being forced to wear an electronic ankle bracelet and “quarantine” at home like a criminal.
Health authorities announced that the measures, scheduled to commence on July 15, are absolutely necessary to stop the spread, flatten the curve, and achieve a “zero COVID” policy of no more “cases” of the disease.
Communist China has a “zero COVID” policy as well, which is why the Chinese Communist Party (CCP) imposed an indefinite lockdown in Shanghai after a few people tested “positive” using the fraudulent PCR test.
“Zero-COVID’s premise is to track down every single infection and thus achieve control and maximum suppression,” reports Reclaim the Net. “In doing so, the authorities deploy a variety of tracing and tracking technology, border closures, and quarantine, as well as strict lockdowns.”
Living with the virus works much better than trying to achieve the impossibility of covid zero
For more than two years now, China and Hong Kong have had a covid zero policy in place, but the measures have done nothing to stop people from testing positive.
A recent “spike” of the disease in Hong Kong, where new cases reached 2,000 per day, prompted the added measure of ankle bracelets, which we are told will stop the virus in its tracks.
“The policy – the opposite of which is ‘living with the virus’ has come under criticism as ultimately failing to deliver on its goal, and being harmful to the economy and people’s health outside of coronavirus concerns,” Reclaim the Net adds.
Unfortunately for Hong Kong, Lo Chung-mau, the country’s health secretary, is a full-fledged covid zero cultist who claimed in the past that “living with the virus will get us all killed.”
How the new bracelets will function and how long people will have to wear them remains unknown. Those details are sure to come once Lo and his fellow covid believers come up with a strategy.
Back in 2020, Hong Kong forced people to wear tracking wristbands as well as quarantine for two weeks. Since that imposition clearly failed, now they are trying ankle bracelets instead, and probably many more weeks of quarantine than just two.
“The wristbands contained a QR code and were paired with a phone app, and were designed to track people’s movements,” reports explain. “Some Hong Kong residents who test positive are directed to quarantine in special facilities, while others are allowed to do this at home.”
Fortunately for the West, there are no such measures being imposed, especially this late in the game when covid is clearly over and done. Perhaps China and Hong Kong will eventually come to the realization that trying to eliminate covid entirely is a fool’s game and stop tyrannizing their people? [MORE] [the answer is the statist’s question]
IsrAlien Authorities Caught Hiding Children's COVID Injection Injuries. Leaked documents show government keeping data from public while approving children’s boosters
/From [HERE] Children aged 5-11 are suffering vaccine injuries, including neurological adverse events, at about 6 times the rate of 12-17 year old children.
Raw data - 2x the injury rate of teens
Israel’s Ministry of Health commissioned a study analyzing reports of adverse events from Pfizer's COVID vaccine to the nation’s vaccine database, known as the Nahlieli system, between December 2021 and May 2022. The research team was headed by Professor Matti Berkowitz, director of the Clinical Pharmacology and Toxicology Unit at Assaf Harofeh Hospital (Shamir).
In raw numbers, Berkowitz found that children in the 5-11 age group had twice as many adverse events following the Pfizer shot as children in the 12-17 age group. That doubling of vaccine injuries is, in itself, extremely disturbing, and should have been immediately brought to the attention of the nation’s parents.
Worse - 6x the injury rate of teens
Unfortunately, the doubling of adverse events is only the beginning of the bad news. Dr. Yaffa Shir-Raz, a health and risk communication researcher at the University of Haifa and at Reichman University (IDC Herzliya), notes that the 2-dose immunization rate for 5-11 year olds is less than 18%, while older children have rates of 55-72% (3-4 times higher).
All things being equal, the young children would thus be expected to have ⅓-¼ of the number of adverse events experienced by the older children, not twice as many. This means that the adverse event rate for young children is actually 6-8 times that of the older children, i.e., at 600-800% of the baseline injury rate!
While there are slightly more children in the 5-11 year old group than in the 12-17 age group, it does not come close to accounting for the mind blowing rate increase in the younger group.
New vaccine injuries not included in Pfizer's leaflet
The findings by Professor Berkowitz were presented to the Ministry of Health’s Department of Epidemiology about three weeks ago, in early June 2022, together with graphs depicting the severity of the data, broken down by injury types, as well as additional alarming information:
. . . the team identified and characterized neurological symptoms that were not previously known and are not mentioned in the physician's leaflet of Pfizer's Comirnaty vaccine, including Hypoesthesia (partial or complete decrease in skin sensitivity), Paraesthesia (abnormal skin sensation such as numbness, tingling, stinging or burning), tinnitus, dizziness and more. [Emphasis added].
Changes to menstrual cycle are long-lasting
Dr. Shir-Raz reports that Pfizer representatives have claimed to have “no knowledge of long-term adverse events.” The research team found, however, that many side-effects of the vaccine are indeed long-term. In the case of changes to the menstrual cycle, 90% of the women reported the change to be long-lasting. Thus,
the research team made it clear to the Department of Epidemiology that Pfizer needed to be notified regarding the long-term adverse events identified. [Emphasis added].
Pfizer should be informed, but not the public?
What you don’t know can hurt you
When some three weeks passed without the Health Ministry making these findings public, those privy to the information became concerned that parents were not being given the information necessary to act with “informed consent” in determining whether to inject their own children.
Leaked documents
The concerned individuals then leaked the data and graphs, which eventually came into the hands of the Professional Ethics Front, an independent Israeli group of physicians, lawyers, scientists, and researchers, who “aim to address the ethical issues related to the COVID-19 crisis in Israeli society.” This watchdog group addressed a letter and follow up correspondence to the official State Comptroller of Israel, Matanyahu Englman, a Knesset appointee charged with overseeing the legality and ethical conduct of public sector institutions:
The findings have been brought to our attention, and they are serious and indicate a risk to children, and in particular to young children aged 5-11 …
The group argued that the information should be disclosed, even if the data is still expected to go through additional analysis,
Out of fear that there is a blatant violation of parents' right to informed consent, and because it constitutes gross negligence, and puts children and infants at risk”.
Too busy to respond?
Despite Israeli law making it clear that the State Comptroller act independently of the executive branch, they have not responded in any way to the group’s requests, prompting the group to file a Freedom of Information Request (FOIA) to get the full report to the public with an acknowledgement of its authenticity. This delay in response comes even as Israel has just approved booster shots for young children and stands poised to add babies and toddlers to the COVID vaccination schedule.
Matches previous reports on danger to small children
This passive monitoring analysis (based on reports initiated by parents) matches the alarming findings from an active monitoring study of adverse events in children aged 5-11 in Israel (tracking every child in the study), which “also demonstrated acute safety signals.”
Matches findings of danger to babies
If small children are having difficulty absorbing the contents of the COVID vaccines, one might expect babies and toddlers to also face dangers from the injections That is just what Dr. Shir-Raz found in an analysis she conducted with her colleague Ranit Feinberg, of Pfizer data on children under 4:
. . . contrary to the FDA's briefing document claiming that the majority of adverse events in Pfizers' clinical trial were non-serious – at least 58 cases of life-threatening side effects in infants under 3 years old who received mRNA vaccines were reported. For some, it is unclear if they survived …
Shir-Raz found the most common serious adverse events to be
life-threatening bleeding, anaphylactic shock, anticholinergic syndrome, encephalitis, hypoglycemia and neuroleptic syndrome. In most of the reported cases, these are multi-system injuries.
In one egregious case, with no indication of whether the baby was enrolled in a Pfizer experiment and lacking any other explanation about how a baby just a few weeks old received the COVID shot, Shir-Raz reports,
"Chest pain; cardiac arrest; Skin cold clammy". This short description of a cardiac arrest, which occurred one hour after receiving a Pfizer-BioNTech COVID-19 vaccine, is taken from the VAERS system - the US Vaccine Adverse Event Reporting System (case number 1015467), and it does not refer to an elderly person, nor to a young adult, or even a teenager. It is hard to believe, but this report refers to a two-month-old baby.
Ominously, this infant’s outcome is labelled unknown.
This case was reported as serious with seriousness criteria-life threatening from HA. No follow-up attempts possible. No further information expected.
Macron's Minority Government Defeated on Genocidal "Vaccine" Passports
/From [HERE] French President Emmanuel Macron suffered a humiliating setback in parliament after his vaccine passport scheme was defeated.
Macron's minority government wanted to extend the policy whereby anyone entering France has to show proof of vaccination or a negative Covid test.
However, the right-wing populist National Rally (RN), the hard-left La France Insoumise (LFI) and the right-wing Republicains (LR) all united to vote against the policy.
Macron's government lost the vote by a margin of 219 votes to 195.
"The bill's defeat was met with wild cheering and a standing ovation from opposition lawmakers, in footage that was widely circulated on social media," reports the Telegraph.
The bill was one of the first put to parliament by the new minority government, highlighting how Macron will find it incredibly difficult to get new laws passed in the country. Elisabeth Borne, the French Prime Minister, condemned the vote.
"The situation is serious. By joining together to vote against the measures to protect the French against Covid, LFI, LR and RN prevent any border control against the virus. After the disbelief on this vote, I will fight so that the spirit of responsibility wins in the Senate," she tweeted.
As we previously highlighted, the French Minister of Health admitted that vaccine passports are a "disguised" form of mandatory vaccines, despite President Macron claiming vaccine mandates "will not be compulsory."
On the first day the new program was in place, police in Paris were visibly patrolling bars and cafes demanding customers show proof they've had the jab.
Mark Zuckerberg Caught on Video Warning COVID Injections are experimental and 'we don’t know the long-term side-effects of modifying people’s DNA and RNA and if it causes mutations, other risks'
/From [HERE] A leaked video of Meta Platforms, Inc. CEO Mark Zuckerberg showed him cautioning his inner circle of the unproven effects of the Wuhan coronavirus (COVID-19) vaccines he called “experimental gene technology.”
Aware of the unsubstantiated curing effects and potential dangers of the vaccines, the tech giant chief felt compelled to alert his people regarding the shots on July 16, 2020 – five months before the initial rollout of COVID-19 vaccines.
“I just want to make sure that I share some caution on this [vaccine] because we just don’t know the long-term side-effects of basically modifying people’s DNA and RNA to directly encode in a person’s DNA and RNA basically the ability to produce those antibodies and whether that causes other mutations or other risks downstream,” Zuckerberg said in the leaked video, which was taken during an internal meeting at Facebook (FB).
Project Veritas founder James O’Keefe noted during the release of the video last year that Zuckerberg would have been “censored on the platform” if what he said to his staff in July was posted on Facebook. He would be “basically violating his own code of conduct,” O’Keefe said.
“It is yet another case of ‘one rule for thee, and another for me’ that the elite use to control the masses,” news website News Punch said.
Back in 2019, the media magnate spoke in front of students at Georgetown Universityabout the importance of protecting free expression. He highlighted his belief that giving everyone a voice gives power to the powerless and pushes society to be better over time– a belief that is at the core of Facebook, he said.However, the freedom to express has been throttled on Facebook and the other major social media platforms in recent years.
Meta had censored 20 million posts since start of pandemic
Last year, FB and Instagram (IG), which is also under the Meta Platforms umbrella, banned major groups, accounts and IG pages for speaking out and raising concerns and doubts about the vaccines’ adverse effects.
According to a Coordinated Inauthentic Behavior Report last year, FB removed 65 of its own and 243 IG accounts for spreading “misinformation” about the COVID-19 vaccines. They have removed more than 20 million individual posts since the start of the pandemic.
In an interview with CBS in August last year, TV anchor Gayle King asked Zuckerberg to release information on how many people have viewed and shared FB posts containing misinformation about the COVID-19 vaccine.
He admitted that FB has removed millions of posts containing misinformation from their website, but failed to answer when pressed by the host on how many people viewed or shared these posts.
“I think, to some degree, there are also different definitions that people have over what misinformation is. A lot of the stuff that’s actually the hardest for us to really address is not what I would call ‘misinformation’ but instead another category that I would call ‘hesitancy,'” he said at the time. (Related: House Republicans demand Zuckerberg surrender all communications with Fauci over covid-19 and “vaccine hesitancy” censorship.)
One of the well-known personalities that have been banned from social media is Robert F. Kennedy Jr., an environmental lawyer who emerged as one of the most influential voices during the early days of the pandemic.
Kennedy Jr., who describes himself as a vaccine safety advocate, was not impressed with Bill Gates’ track record of pushing vaccines on vulnerable populations, causing serious health problems in some cases. Subsequently, Meta “fact-checkers” banned Kennedy Jr. from Instagram for speaking out about vaccine safety.
Father’s 20-Year Battle on Behalf of Vaccine-Injured Son Exposes Travesty of Liability-Free Vaccines
/From [CHD] In a riveting legal battle spanning two decades, William Yates Hazlehurst (“Yates”) on Feb. 2, 2022, became the first vaccine-injured person with a diagnosis of autism to reach a jury since the National Childhood Vaccine Injury Compensation Act of 1986 (the Vaccine Act) became law.
In a medical malpractice case filed in the Madison County Circuit Court in Tennessee, attorneys for Yates argued the clinic and physician who administered Yates’ vaccines, including the measles-mumps-rubella (MMR) vaccine on Feb. 8, 2001, should be held liable for medical malpractice and the neurological injuries Yates developed after being vaccinated.
Although the jury decided in favor of the physician — who Yates’ father said failed to adequately inform the parents of the risks of vaccinating Yates while he had an active ear infection — the case exposed major flaws in a system designed to protect children and shield pharmaceutical companies and physicians from liability for vaccine injuries.
“In the fight to end the autism epidemic, we were all hoping for the one knockout punch that would bring the truth to light and help end the autism epidemic,” Yates’ father, Rolf Hazlehurst, said.
“This medical malpractice trial was the only opportunity in the last 35 years for a jury to hear evidence in a court of law regarding whether a vaccine injury can cause neurological injury, including autism.”
Hazlehurst, who is a senior staff attorney for Children’s Health Defense (CHD), said “unless the Vaccine Act is repealed, my son is probably the only vaccine-injured child with a diagnosis of autism who will ever reach a jury.”
The Hazlehurst case was a medical malpractice case against the doctor who administered the pediatric vaccines that, in the opinion of the world’s top experts, sent Yates, now 22, spiraling into the depths of severe, non-verbal autism.
Although the case was originally filed in 2003, it didn’t receive its day in court for 19 years because a separate case involving Yates’ injury first had to work its way through the National Vaccine Injury Compensation Program (NVICP).
When Yates’ medical malpractice case was finally heard, the trial exposed alarming evidence about autism and vaccines, the low standard of care practiced by physicians administering pediatric vaccines and financial conflicts of interests between pharmaceutical companies that manufacture vaccines and government agencies entrusted with vaccine safety.
During the trial, the world’s top experts in the field of autism and mitochondrial disorderexplained how the administration of “routine” childhood immunizations can cause autism, brain injury, and many other disorders.
According to the National Institute of Mental Health, autism is a neurological and developmental disorder that affects how people interact with others, communicate, learn and behave. Symptoms can be severe and usually manifest before a child turns 3, which coincides with the age children receive the most childhood vaccines.
Increasing evidence indicates a significant proportion of individuals with autism have concurrent diseases such as mitochondrial dysfunction, abnormalities of energy generation, gastrointestinal abnormalities and abnormalities in the regulation of the immune system.
Yates’ medical malpractice trial illuminated how vaccines can cause autism in children with mitochondrial disorder and showed how the Vaccine Act — which is designed to ensure informed consent and compensation to injured children — is an abject failure because it’s largely unenforceable.
Yates was normal until he received his 12-month vaccines
During the first year of his life, Yates developed typically and met all of his developmental milestones.
“He was a happy, healthy and normal child,” his father said.
After his 6-month shots, Yates experienced a severe screaming episode approximately 24 hours after receiving the DTaP, Prevnar, Hib and Hep B vaccines.
In the days following his vaccinations, Yates began to experience seizure-like shaking episodes.
But his parents didn’t realize their son’s symptoms were consistent with a severe vaccine adverse reaction because they were not given a Vaccine Information Statement (VIS) at their pediatrician’s office.
According to the Centers for Disease Control and Prevention (CDC), a VIS is an information sheet produced by the CDC that explains both the benefits and risks of a vaccine to recipients.
“Federal law requires that healthcare staff provide a VIS to a patient, parent or legal representative before each dose of certain vaccines,” the CDC website states.
Instead of providing the VIS, Yates’ physician told his parents any adverse event to a vaccine would be “almost immediate” — within 5 to 15 minutes after vaccination.
Before Yates’ first birthday, his mother and aunt took him to the doctor because he had been sick, and his parents wanted to make sure it was okay for Yates to have a birthday party.
Hazlehurst told The Defender this appointment was not a scheduled well-child check. It was a sick visit. At the appointment, Yates was diagnosed with an ear infection and prescribed an antibiotic.
As the pediatrician turned to leave, he stated Yates would receive his shots, as it was close to his first birthday. A woman returned to the room who portrayed herself to be a nurse, but Hazlehurst later found out was only a medical assistant.
Yates’ mother asked the “nurse” whether their son should receive his shots despite being sick and was told he should.
Once again, they were not given a VIS form informing them of the risks of vaccinating Yates while he had a fever and an active ear infection.
“By administering vaccines to a sick child, the doctor and his clinic could charge a “modified double bill” Hazlehurst said.
That day, on Feb. 8, 2001, Yates received the MMR, Prevnar, Hib and Hep B vaccines. Twelve days later, Hazlehurst said his son experienced a high fever, rash and vomiting consistent with a vaccine adverse reaction.
Hazlehurst called the clinic where his son received his vaccine and talked to the doctor on call who asked him which vaccines Yates received. Hazlehurst responded, “whatever you get when you’re a year old.”
Hazlehurst was told his son was having an adverse reaction to the antibiotic and the doctor wrote him a prescription for a different antibiotic and an anti-fungal medication.
Soon after, Yates began to lose the skills he once had and began developing abnormally. He lost his speech, started running wild, was constantly on the go and would knock things off the table.
“He was visually ‘stimming’ off the falling objects and running with his head down for the visual stimulation,” Hazlehurst said.
He explained:
“It was not like he got the shots and boom, the next day he was autistic. That’s not the way it happened. The mitochondria produce the energy to the connecting tissue in the cells in the brain, and if they don’t get enough energy for a short period of time (as short as 6 seconds), cellular death occurs.
“The brain keeps developing, but it cannot develop normally because the connecting cellular tissue has been damaged. That’s why it takes time to manifest. It’s like watching grass grow. It’s happening, but you don’t realize it’s happening.”
Yates’ condition worsened. He developed an obsession with spinning objects, became a picky eater, started hand-flapping and toe-walking, became unable to sleep and exhibited gastrointestinal and multiple other medical and neurodevelopmental issues, Hazlehurst said.
On June 3, 2002, Yates was diagnosed with autism spectrum disorder.
Hazlehurst searches for answers to his son’s autism
According to federal law, there are specific recording requirements for vaccine medical records, and healthcare providers must provide records to a parent upon request.
Hazlehurst, on June 21, 2002, requested a copy of his son’s original vaccine records so other physicians could evaluate, diagnose and treat Yates.
Hazlehurst had questions about the American Academy of Pediatrics’ standard of care and wanted to know why his son was vaccinated while he was sick with a fever.
In response to Hazlehurst’s request and questions about Yates’ care, the pediatrician rushed out of the room and called his attorney, Hazlehurst said.
The doctor and clinic denied Hazlehurst’s requests to review and receive copies of his son’s original vaccine records, forcing him to petition the court for Yates’ records.
The court granted the request, and the local sheriff’s department seized Yates’ medical records from the doctor’s clinic.
Hazlehurst quickly realized there were problems with his son’s vaccine record, which was on an unsigned consent form that had a billing code sticker placed over the language regarding the risks and benefits of vaccines and vaccine information materials.
Hazlehurst said he never received a VIS form and Yates had been vaccinated without informed consent.
Hazlehurst files claim with the NVICP for son’s vaccine injury
Hazlehurst, like many parents of vaccine-injured children, pursued a claim with the NVICP as federal law requires. The process took nine years — from 2002 to 2011.
In order to bring a case in a court of law, the parents of a vaccine-injured child must first file their case with the NVICP.
The NVICP is a special, no-fault tribunal housed within the U.S. Court of Federal Claims that handles injury claims for 16 federally recommended vaccines. To date, the court has awardedmore than $4 billion to thousands of people for vaccine injuries.
In the NVICP, America’s legal system is replaced by a “special master.” The special masters who review claims are government-appointed attorneys, many of whom are former U.S. Department of Justice (DOJ) attorneys.
Under the NVICP, the parents of vaccine-injured children are forced to sue the secretary of the U.S. Department of Health and Human Services (HHS) for compensation. HHS is represented by DOJ attorneys.
It is exceptionally difficult to obtain compensation within the NVICP, Hazlehurst said. The proceedings are often turned into drawn-out, contentious expert battles and the backlog of cases is substantial. Because of this, a single case can drag on for over a decade.
Payouts, including attorneys’ fees, are funded by a 75-cent tax per vaccine. There is a $250,000 cap on pain and suffering and death benefits.
The Vaccine Act established the NVICP, and the 2011 U.S. Supreme Court decision Bruesewitz et al v. Wyeth et al later guaranteed vaccine manufacturers, doctors and other vaccine administrators almost always have no legal accountability or financial liability in civil court when a government-recommended or mandated vaccine(s) causes permanent injury or death, Hazlehurst said.
The NVICP ultimately denied Yates’ claim, but his case against HHS became a central part of the U.S Supreme Court’s decision in Bruesewitz v. Wyeth.
Yates’ case in the NVICP was part of the Omnibus Autism Proceeding (OAP), in which 5,400 claims submitted to the NVICP were consolidated to determine if vaccines cause autism and if so, under what conditions.
“HHS whittled down the thousands of cases to six “test cases,” one of which was Yates’ case,” Hazlehurst said. “If HHS could find a way to deny NVICP compensation to the test cases, the agency would be able to deny compensation to all 5,400 families.”
Hazlehurst said HHS and the DOJ “took advantage of the fact that the rules of evidence, discovery and civil procedure mechanisms available in a regular court do not apply in the so-called vaccine court, and perpetrated fraud upon the special masters, the Court of Appeals for the D.C. Circuit and ultimately, the U.S. Supreme Court.”
The special masters on Feb. 12, 2009, in the so-called vaccine court, denied Yates’ petition for compensation and those of the five remaining OAP “test cases” involving children who developed autism after receiving their pediatric vaccines.
HHS makes key concession in Hannah Poling case
The potential fourth test case — Hannah Poling’s — was quietly conceded in 2007, in a corrupt coverup to conceal the opinion of the HHS expert witness, Dr. Andrew Zimmerman, the world’s leading expert in autism research, Hazlehurst said.
When Poling was 19 months old, she was vaccinated against nine diseases at one doctor’s visit: measles, mumps, rubella, polio, varicella, diphtheria, pertussis, tetanus and Haemophilus influenzae type b. In total, she received five vaccines.
Prior to receiving her vaccines, Poling was described as normal, happy, healthy, interactive, playful and communicative. But two days after being vaccinated, she was lethargic, irritable and febrile, and within 10 days she developed a rash consistent with vaccine-induced chicken pox.
Over the course of several months, Poling stopped eating, didn’t respond when spoken to, began showing signs of autism, developed neurological and psychological disorders and was diagnosed with encephalopathy caused by an underlying mitochondrial disorder.
In 2003, Poling’s father, Jon, a physician and trained neurologist, and mother, Terry, an attorney and nurse, filed an autism claim against HHS under the NVICP for their daughter’s injuries.
Five years later, the government settled the case before trial and in essence had it sealed.
During the OAP, in the Poling case, the government quietly conceded vaccines caused “regressive encephalopathy with features of autism spectrum disorder.”
According to CBS News, Poling received more than $1.5 million dollars for her life care, lost earnings and pain and suffering for the first year alone. After the first year, the family was supposed to receive more than $500,000 per year to pay for Poling’s care, which is estimated to amount to $40 million over her lifetime.
Jon Poling on March 6, 2008, said, “the results, in this case, may well signify a landmark decision with children developing autism following vaccinations.”
Prior to the Poling case, federal health agencies and professional organizations had reassured the public vaccines didn’t cause autism. The Poling case challenged that narrative, which is why the case was conceded and in essence sealed.
HHS’ concession that Poling developed autism as a result of a vaccine injury briefly became international news. Yet, only a handful of people knew why the government conceded Hannah’s case.
When news of the concession in Poling v. HHS was made public in March 2008, Dr. Julie Gerberding, then-director of the CDC, in an interview with CNN’s Dr. Sanjay Gupta said:
“We all know that vaccines can occasionally cause fevers in kids, so if a child was immunized, got a fever, had other complications from the vaccines, then if you are predisposed with a mitochondrial disorder, it can certainly set off some damage — some of the symptoms can be symptoms that have characteristics of autism.”
If HHS had not conceded her case, the truth as to how vaccines cause autism in some children with an underlying mitochondrial disorder would have been exposed by the world’s leading expert witnesses in the spotlight of the OAP, Hazlehurst said.
The concession document in the Poling case states:
“The vaccinations Hannah received on July 19, 2000, significantly aggravated an underlying mitochondrial disorder, which predisposed her to deficits in cellular energy metabolism, and manifested as a regressive encephalopathy with features of autism spectrum disorder.”
Zimmerman was an expert witness for the government defending vaccines in the NVICP. In 2007, during the hearing in the first test case, he told the government vaccines could cause autism in “exceptional” cases, but said the government later hid that information and misrepresented his expert opinion.
In a 2018 letter, Robert F. Kennedy, Jr., CHD chairman and chief legal counsel, and Hazlehurst meticulously described the DOJ’s fraud pertaining to the misrepresentation of Zimmerman’s opinions in the OAP and requested an investigation.
“The Office of Inspector General passed the buck to the DOJ Department of Ethics,” Hazlehurst said. “The DOJ investigated itself and wrote a highly misleading letter absolving itself of any wrongdoing.”
Zimmerman said in a signed affidavit:
“Shortly after I clarified my opinions with the DOJ attorneys, I was contacted by one of the junior DOJ attorneys and informed that I would no longer be needed as an expert witness on behalf of H.H.S. The telephone call … occurred after the above-referenced conversation on Friday, June 15, 2007, and before Monday, June 18, 2007. To the best of my recollection, I was scheduled to testify on behalf of H.H.S. on Monday, June 18, 2007.”
As a result of his firing, Zimmerman was not present for the Hazlehurst OAP proceedings, which allowed DOJ attorneys to misrepresent Zimmerman’s statements related to a separate autism case and apply them to all cases of autism, including Yates’ case.
Over the years Hazlehurst has repeatedly stated, “I want to be very clear, neither the Polings nor Dr. Zimmerman did anything wrong.”
“But,” he added, “if I did to a criminal, in a court of law, what the United States Department of Justice did to vaccine-injured children, I would be disbarred and I would be facing criminal charges.”
Zimmerman did testify as an expert witness on behalf of Yates in the medical malpractice case filed against Yates’ doctor, which was finally heard by a Tennessee court in February 2022.
Research by Zimmerman and others determined that at least 30%-40% of children with a diagnosis of regressive autism suffer from a mitochondrial disorder, which is a condition with which Yates was later diagnosed.
Yates in ‘perfect position’ to file lawsuit after exhausting remedies in NVICP
After exhausting all remedies under the NVICP — a process that took 25 years — the legal floodgates were then open, Hazlehurst said.
But because no one could sue the vaccine manufacturer, the only vaccine-injured child — out of thousands of cases originally included in the OAP — left with legal standing was Yates Hazlehurst and his claim of medical malpractice against the pediatrician who oversaw the administration of his vaccines.
Ultimately, the same medical experts, including Zimmerman and Dr. Richard Kelley, former director of the Genetics Department at Johns Hopkins Medical Institute — whose testimony HHS and the DOJ relied on in the Poling concession — concluded that what happened to Hannah Poling is what also happened to Yates Hazlehurst.
In an affidavit which was not admissible in the 2022 medical malpractice trial, Kelley stated:
“I also find, with a high degree of medical certainty, that the set of immunizations administered to Yates at 11 months while he was ill was the immediate cause of his autistic regression because of the effect of these immunizations to further impair the ability of his weakened mitochondria to supply adequate amounts of energy for the brain, the highest energy-consuming tissue in the body.”
Zimmerman’s expert opinion on the cause of Yates’ neurological condition was consistent with Kelley’s opinion. [MORE]
New Research Demonstrates Wearing Masks May Make You Sick and Accumulating Research Shows Mask Mandates Didn't Lower the Spread of COVID, whether community spread of COVID was low or high
/STORY AT-A-GLANCE
Using CDC data, no significant differences were found in COVID-19 case growth between states with or without mask mandates, during periods of low or high transmission
The widespread use of masks did not reduce COVID-19 transmission in Europe, and a moderate positive correlation was found between mask usage and deaths in Western Europe
An update to a CDC study on school mask mandates, using nearly six times more data, found no significant relationship between mask mandates in U.S. schools and COVID-19 case rates
In Kansas, counties with a mask mandate had significantly higher COVID-19 case fatality rates than counties without a mask mandate
One way masks cause harm may be the “Foegen effect” — the idea that deep re-inhalation of droplets and virions caught on facemasks might make COVID-19 infection more likely or more severe
From [MERCOLA] and [PDF] During the COVID-19 pandemic, 80% of U.S. states mandated masks to slow the spread of SARS-CoV-2, but accumulating research shows mask mandates and use do not lower the spread of the virus.1 While rules requiring masks did increase compliance, they didn't translate to lower transmission growth rates, whether community spread of SARS-CoV-2 was low or high.
Even before COVID-19 was declared a pandemic, mask mandates were put in place without ever properly evaluating efficacy, but that didn't stop them from dividing communities and being used as a form of virtue signaling and a visible reminder of compliance with the "new normal."
Now, with research showing not only that masks don't protect you but may actually make you sick, the rationale behind their widespread mandated usage must be questioned.
Mask Mandates Didn't Lower COVID-19 Cases
Using CDC data, researchers with the University of Louisville calculated total COVID-19 case growth and mask use for the U.S. No significant differences were found in case growth between mandate and non-mandate states during periods of low or high transmission.
"Surges were equivocal," they noted, concluding, "Mask mandates and use are not associated with slower state-level COVID-19 spread during COVID-19 growth surges."2 While stating that their findings "do not support the hypothesis that SARS-CoV-2 transmission rates decrease with greater public mask use," they did note that "masks may promote social cohesion as rallying symbols during a pandemic."3
Similarly dismal results from mask mandates were demonstrated in Europe. A study published in Cureus analyzed data from 35 European countries, including morbidity, mortality and mask usage, over a six-month period. The researchers noted:4
"Mask mandates were implemented in almost all world countries and in most places where masks were not obligatory, their use in public spaces was recommended … These mandates and recommendations took place despite the fact that most randomized controlled trials carried out before and during the COVID-19 pandemic concluded that the role of masks in preventing respiratory viral transmission was small, null, or inconclusive."
When the data were analyzed, the study also revealed that the widespread use of masks did not reduce COVID-19 transmission. Worse, a moderate positive correlation was found between mask usage and deaths in Western Europe, which "suggests that the universal use of masks may have had harmful unintended consequences."5
Mask Mandates in Schools Didn't Reduce COVID-19 Cases
As part of the government sponsored propaganda campaign, a widely cited CDC study, published in October 2021, reported that counties without school mask mandates had larger increases in COVID-19 case rates in children after the start of school compared with counties that had school mask mandates.6
The study was used to support school mask mandates, but a team of researchers revisited the research, incorporating a larger sample size and longer study period. The updated study,7 published in May 2022, used nearly six times more data compared to the original study and found no significant relationship between mask mandates in U.S. schools and COVID-19 case rates. According to the researchers:8
"We failed to establish a relationship between school masking and pediatric cases using the same methods but a larger, more nationally diverse population over a longer interval. Our study demonstrates that observational studies of interventions with small to moderate effect sizes are prone to bias caused by selection and omitted variables. Randomized studies can more reliably inform public health policy."
On Twitter, surgeon and public policy researcher Dr. Marty Makary pointed out that the CDC's original study appeared to include cherry-picked data and the agency refused to publish an update using the more extensive data:9
"This study demonstrates how the CDC was cherry-picking data to support their school mask dogma. The article states that CDC's MMWR journal rejected publishing this re-analysis. Most likely because it exposed the CDCs salami-slicing of data & use of science as political propaganda."
It should be noted that a previous CDC study found mask requirements for students had little effect on COVID-19 incidence in Georgia schools, while improved ventilation, such as opening a window, reduced cases more than mask mandates for staff and teachers.10
The Foegen Effect: Mask Mandates Increased COVID-19 Deaths
A profoundly important study was conducted by German physician Dr. Zacharias Fögen to find out whether mandatory mask use influenced the COVID-19 case fatality rate in Kansas from August 1, 2020, to October 15, 2020.11 He chose the state of Kansas because, while it issued a mask mandate, counties were allowed to either opt in or out of it.
His analysis revealed that counties with a mask mandate had significantly higher case fatality rates than counties without a mask mandate. "These findings suggest that mask use might pose a yet unknown threat to the user instead of protecting them, making mask mandates a debatable epidemiologic intervention," he concluded.
That threat, he explained, may be something called the "Foegen effect" — the idea that deep re-inhalation of droplets and virions caught on facemasks might make COVID-19 infection more likely or more severe.
“The fundamentals of this effect are easily demonstrated when wearing a facemask and glasses at the same time by pulling the upper edge of the mask over the lower edge of the glasses. Droplets appear on the mask when breathing out and disappear when breathing in.”
"In the "Foegen effect," the virions spread (because of their smaller size) deeper into the respiratory tract. They bypass the bronchi and are inhaled deep into the alveoli, where they can cause pneumonia instead of bronchitis, which would be typical of a virus infection.
Furthermore, these virions bypass the multilayer squamous epithelial wall that they cannot pass into in vitro and most likely cannot pass into in vivo. Therefore, the only probable way for the virions to enter the blood vessels is through the alveoli."12
Wearing Masks Could Be Related to Long COVID
Fögen explained that wearing masks could end up increasing your overall viral load because, instead of exhaling virions from your respiratory tract and ridding your body of them, those virions are caught in the mask and returned. This might also have the effect of increasing the number of virions that pass through the mask, such that it becomes more than the number that would have been shed without a mask.
The fact that "hypercondensed droplets and pure virions in the mask might be blown outwards during expiration, resulting in aerosol transmission instead of droplet transmission" is another issue that could make transmission worse instead of better, and the use of "more protective" masks could also backfire, making COVID-19's long-term effects worse. Fögen explained:13
"The use of "better" masks (e.g., FFP2, FFP3) with a higher droplet-filtering capacity probably should cause an even stronger "Foegen effect" because the number of virions that are potentially re-inhaled increases in the same way that outward shedding is reduced.
Another salient point is that COVID-19-related long-term effects and multisystem inflammatory syndrome in children may all be a direct cause of the "Foegen effect." Virus entry into the alveoli and blood without being restricted to the upper respiratory tract and bronchi and can cause damage by initiating an (auto) immune reaction in most organs."
Clear Risks of Prolonged Mask Use
Two expert reports spoke out against the use of masks for children in 2021. The first, a psychology report,14 stated that masks are likely to be causing psychological harm to children and interfering with development.15 "The extent of psychological harm to young people is unknown," the report stated, "due to the unique nature of the 'social experiment' currently underway in schools, and in wider society."16
The second report focused on health, safety and well-being,17 noting potential permanent physical damage to the lungs caused by fibrosis from inhalation of fibrous nanoparticles.
"There are real and significant dangers of respiratory infection, oral health deterioration and of lung injury, such as pneumothorax, owing to moisture buildup and also exposure to potentially harmful levels of an asphyxiant gas (carbon dioxide [CO2]) which can cause serious injury to health," the authors explained.18
Normally, the CO2 then dissipates into the air around you before you take another breath. In the open air, carbon dioxide typically exists at about 400 parts per million (ppm), or 0.04% by volume.
The German Federal Environmental Office set a limit of CO2 for closed rooms of 2,000 ppm, or 0.2% by volume. If you're wearing a facemask, the CO2 cannot escape as it usually does and instead becomes trapped in the mask. In a study published in JAMA Pediatrics, researchers analyzed the CO2 content of inhaled air among children wearing two types of masks, as well as wearing no mask.19
While no significant difference in CO2 was found between the two types of masks, there was a significant elevation when wearing masks compared to not wearing them. CO2 in inhaled air under surgical and filtering facepiece masks came in between 13,120 ppm and 13,910 ppm, "which is higher than what is already deemed unacceptable by the German Federal Environmental Office by a factor of 6," the researchers noted.20
Also important, this level was reached after only three minutes, while children wear masks at school for a mean of 270 minutes at a time. Even the child who had the lowest measured CO2 level had a measurement three-fold greater than the closed room CO2 limit of 0.2%. However, younger children appeared to have the highest CO2 values; a level of 25,000 ppm was measured from a 7-year-old wearing a facemask.21
Bacterial Infection Risk, Problems With Social Learning
The full consequences of prolonged mask use are only beginning to be understood. The University of Louisville researchers noted, however, that using a mask for more than four hours per day "promotes facial alkalinization and inadvertently encourages dehydration, which in turn can enhance barrier breakdown and bacterial infection risk."22 Other reported adverse effects include:23
After a lawsuit was brought by Leslie Manookian's Health Freedom Defense Fund (HFDF), U.S. District Judge Kathryn Kimball Mizelle finally voided the CDC's U.S. mask mandate on airplanes and public transit in April 2022.24 The U.S. Department of Justice (DoJ) has appealed the court order,25however, making it clear that they don't intend to give up on mask mandates without a fight. In response, HFDF issued the following statement:26
"DoJ's statement [that it would appeal] is perplexing to say the least and sounds like it comes from health policy advocates not government lawyers. The ruling by the US District Court ruling is a matter of law, not CDC preference or an assessment of 'current health conditions.'
If there is in fact a public health emergency with clear and irrefutable science supporting CDC's mask mandate, does it not warrant urgent action? Why would DoJ and CDC not immediately appeal?
HFDF is left with no option but to conclude that the Mask Mandate is really a political matter and not at all about urgent public health issues or the demands of sound science. While DoJ and CDC play politics with Americans' health and freedoms, HFDF trusts individual Americans to make their own health decisions.
HFDF is confident that Americans possess ample common sense and education to understand that there are real questions about mask efficacy and risk and that CDC's policy reflects neither."
You can support the Health Freedom Defense Fund and push back against the DoJ and CDC by taking to social media. Please follow and/or like the HFDF on the following platforms, share their content, and invite your followers to do the same:
Instagram: @healthfreedomdefensefund
Twitter: @theHFDF
Facebook: @healthfreedomdefensefund
Telegram: @HealthFreedomDefense
Sources and References
1, 2, 3, 22, 23 MedRxiv May 25, 2021
10 U.S. CDC, Morbidity and Mortality Weekly Report May 21, 2021
14, 16 Psychology Report in respect of Civil Proceedings April 9, 2021
17, 18 Health, Safety and Wellbeing Report in respect of Civil Proceedings April 9, 2021
19, 20, 21 JAMA Pediatrics June 30, 2021
24 US District Court Middle District of Florida Case No: 8:21-cv-1693-KKM-AEP
FDA Grants Emergency Use Authorization for Novavax COVID Shots - Linked to Heart Inflammation, Blood Clots. Biden Purchased $3B of Shots Designed for the Original Variant, which has been Supplanted
/THE BLIGHT HOUSE PROMISES TO LIE TO YOU OVER AND OVER.
From [CHD] The U.S. Food and Drug Administration (FDA) today granted Emergency Use Authorization (EUA)for the Novavax COVID-19 vaccine for adults 18 and over.
The EUA is for the two-dose primary series targeting the original Wuhan SARS-CoV-2 virus — limiting the vaccine’s use, as about two-thirds of Americans already have completed a primary series of either the Pfizer, Moderna or Johnson & Johnson vaccines.
The Centers for Disease Control and Prevention (CDC) still needs to sign off on the Novavax vaccine before pharmacies and other healthcare providers can start administering shots.
The vaccine maker’s stock rose 3% earlier today, after Politico reported yesterday that the FDA would likely announce the decision today.
The Biden administration on Monday announced a deal with Novavax to purchase 3.2 million doses of the vaccine.
Under the taxpayer-funded deal — which the U.S. Department of Health and Human Services said was contingent on the vaccine receiving EUA and formal recommendation by the Centers for Disease Control and Prevention — the U.S. government will provide the vaccine to states, jurisdictions, federal pharmacy partners and federally qualified health centers.
Advisors to the FDA last month recommended the agency accept Novavax’s EUA application, but the agency delayed issuing the authorization pending FDA review of the Maryland-based company’s manufacturing process.
Novavax already is available in other countries, including Canada and Australia, under the name Nuvaxovid.
The Novavax vaccine relies on a protein-based technology used for decades, leading some media outlets to portray it as a “traditional” vaccine compared with other COVID-19 vaccines that use newer technologies.
Politico reported last month that FDA committee members expressed interest in making available a vaccine that uses a different technology than the mRNA vaccines widely used in the U.S., “in hopes of convincing unvaccinated holdouts to change their minds.”
According to Politico, Novavax “may appeal to the sliver of the population allergic to components of the messenger RNA vaccines developed by Pfizer-BioNTech and Moderna, or who are skeptical of those shots’ newer technology.”
But according to Dr. Meryl Nass, an internist with a special interest in vaccine-induced illnesses, chronic fatigue syndrome and toxicology, the media’s portrayal of Novavax as a more traditional vaccine is not accurate.
Nass, a member of the Children’s Health Defense (CHD) scientific advisory committee, pointed out that the Novavax shot contains a novel adjuvant, Matrix-M, “so it is not really an old-fashioned shot.”
Nass raised safety concerns specific to the adjuvant, while others voiced concerns about Novavax being linked to heart inflammation and blood clots, and the fact that the vaccine was designed for use against the original Wuhan strain of SARS-CoV-2 — not the various Omicron variants that are dominant today.
How does Novavax differ from other COVID vaccines used in U.S.?
Novavax is a subunit protein vaccine. It uses the spike protein, which it delivers directly to the host cell, from the viral coat of the SARS-CoV-2 virus, as the antigen — the part of the vaccine that provokes an immune response.
The mRNA-based shots — Pfizer and Moderna — use a lipid nanoparticle to encapsulate the mRNA and usher it into the host cell. Then the host cell’s own machinery produces the spike protein.
“Unlike mRNA vaccines, the spike protein is already premade in the Novavax vaccine, said Dr. Diana Florescu, who led the Novavax clinical trial. “It’s a shortcut. All the synthesis happens outside the body and we just give the end product: the spike protein.”
Johnson & Johnson’s Janssen COVID-19 shot is a viral vector vaccine. It also causes cells to produce the spike protein, but in a different way than the mRNA shots. It uses a virus called adenovirus, familiar as a common cause of respiratory infections.
The DNA in the adenovirus is modified so that when it enters the host cell, it causes the cell’s own machinery to produce the spike protein.
The adenovirus is also modified so it cannot replicate itself, which is why it is called a replication-defective recombinant adenoviral vector vaccine.
Adjuvant used in Novavax linked to autoimmune disease
Because Novavax is a protein subunit vaccine, it uses just the spike protein as the antigen rather than the whole pathogen (an inactivated or attenuated virus). Using the whole pathogen would expose the host to the virus’ entire protein coat instead of just one protein.
Protein subunit vaccines are often less immunogenic (less likely to provoke the immune system) than vaccines that use whole pathogens as the antigen, and may not generate a strong enough immune response.
That’s why they require the use of an adjuvant — in this case, Matrix-M — in addition to the antigen to get a stronger immune response.
However, few adjuvants are both potent and non-toxic enough for clinical use.
The proposed primary series for Novavax is two intramuscular injections 21 days apart at the dose level of 5 µg of the recombinant spike protein and 50 µg of the Matrix-M adjuvant.
Matrix-M, originally called QS-21, was one of the saponins derived from Quillaja saponaria, which is the soap bark tree native to Chile.
Some reports point out that the Matrix-M adjuvant — unlike the polyethylene glycol (PEG) lipidused in mRNA vaccines — is not linked to anaphylaxis (a severe allergic reaction), making it more attractive to people who are allergic to PEG.
But according to Nass, while it’s true that Matrix-M — which is not found in any other vaccines in the U.S. — isn’t linked to anaphylaxis, it is linked to autoimmune diseases.
“While touted as a replacement for the PEG lipid found in the mRNA vaccine, Matrix-M is less likely to cause anaphylaxis but more likely to cause autoimmune diseases,” Nass said.
Nass voiced other safety concerns about Novavax technology, including the use of moth cells.
According to the University of Nebraska Medical Center, where Novavax Phase 3 clinical trialswere conducted, the Novavax vaccine uses moth cells to create a nanotechnology version of the COVID-19 spike protein.
Nass said insect cells can be used to grow proteins rapidly. “There is one flu vaccine made the same way: Flublok,” Nass said. Flublok is one of two egg-free flu vaccines licensed for use in the U.S.
“How many insect and viral proteins or other molecules are being injected into you when you get the Novavax vaccine — which is a function of how purified the vaccine is — is unknown,” Nass said.
Novavax still uses the spike protein
The SARS-CoV-2 virus encodes 29 proteins, but Novavax — like Pfizer, Moderna and Johnson & Johnson — chose to target only the spike protein.
As previously reported in The Defender, it is not known if the spike protein itself is safe.
“We have known for a long time that the spike protein is a pathogenic protein,” said Byram Bridle, Ph.D., assistant professor of immunology at the University of Guelph, Ontario. “It is a toxin. It can cause damage to our body if it gets into circulation.”
According to Brian Hooker, Ph.D., CHD’s chief scientific officer, “If you wanted to pick the most toxic protein, you know what represents the highest virulence, the highest amount of damage on the COVID-19 virus? You would pick the spike protein.”
The spike protein “has been consistently shown to create clotting issues in the blood,” Hooker said.
Novavax downplays link to heart issues
According to the briefing document for the Vaccines and Related Biological Products Advisory Committee meeting on June 7, severe local adverse events occurred in 1.2 to 7.2% of Novavax recipients, and systemic adverse events occurred in 2.4% to 12.1% of Novavax recipients.
These adverse events were more frequent after the second dose than after the first dose.
As previously reported in The Defender, there are concerns that Novavax is associated with myocarditis and pericarditis, just like the mRNA vaccines.
Reuters reported that the FDA asked Novavax to “flag” myocarditis and pericarditis as an “important identified risk” in its materials accompanying the vaccine. It is not known if the vaccine maker agreed to do so.
Novavax denied the connection between its vaccine and the reported cases of heart inflammation, claiming that “natural background events” of myocarditis can be expected in any large database.
“Based on our interpretation of all the clinical data supporting NVX-CoV2373 [Novavax]… we believe there is insufficient evidence to establish a causal relationship,” Novavax stated.
Does it work against the Omicron variant?
Like the other COVID-19 vaccines available in the U.S., Novavax’s vaccine was developed against the ancestral Wuhan strain of SARS-CoV-2.
In the FDA’s June 7 briefing document on the vaccine’s efficacy and safety, the FDA stated:
“The study enrollment and efficacy follow-up occurred during December 27, 2020, to September 27, 2021, and mainly when the Alpha variant of SARS-CoV-2 was predominant and prior to the emergence of Delta and Omicron variants.
“Relevant data to assess effectiveness of NVX-CoV2373 [Novavax] against the Omicron variant and sublineages, including observational data from use in other countries where the vaccine has been deployed, are currently unavailable; however, based on the efficacy estimate in the clinical trial of this vaccine, it is more likely than not that the vaccine will provide some meaningful level of protection against COVID-19 due to Omicron, in particular against more severe disease.”
The FDA briefing document also stated that due to the limited length of follow-up, “it is not currently possible to assess sustained efficacy over a period longer than 2 months.”
Bruce Gellin, chief of global public health strategy at the Rockefeller Foundation, was the lone abstaining vote on the FDA committee that voted to recommend Novavax on June 7.
Gellin said he abstained because the committee wasn’t given data on how the vaccine performs against the current Omicron variants, or for how many months its protection lasts.
Will Novavax convince the unvaxxed?
CNET last month reported that more than two years into the pandemic, a majority of Americans (about 67%) are fully vaccinated against COVID-19, and many have been boosted.
A Kaiser Family Foundation poll found 75% of adult Americans self-report that they are already vaccinated.
Meanwhile, those hesitant or opposed to COVID-19 vaccination seem to be firm and consistent in their opinion, according to the Kaiser poll.
Kaiser has tracked the “public’s attitudes and experiences with COVID-19 vaccinations” since December 2020. During that time, the percentage of American adults who answered the poll and said they would “definitely not” get vaccinated ranged from 12% to 17%.
In April, the most recent month reported, 17% of those polled said they would “definitely not” get vaccinated against COVID-19.
Millions of Americans have already been infected with COVID-19 and recovered. As of February 2022, the overall seroprevalence rate (indicating previous COVID-19 infection) in the U.S., determined by random antibody testing, was 57%.
Emails Confirm Vaccine Definition was Changed b/c COVID Shots Aren’t “Vaccines.” [“Immunity” was Removed from Definition. Shots are a “Treatment” and People have a Right to Refuse “Medical Treatment”]
/From [HERE] and [HERE] Newly obtained emails confirm that the Centers for Disease Control and Prevention (CDC) changed its definition for both “vaccine” and “vaccinated” because people were pointing out that definitions didn’t seem to apply to the COVID-19 vaccines.
“The definition of vaccine we have posted is problematic and people are using it to claim the COVID-19 vaccine is not a vaccine based on our own definition,” Alycia Downs, a CDC official, wrote in an email on Aug. 25, 2021, to a colleague.
The definition is located on a page titled Immunization Basics.
“Vaccine” was defined since at least 2011 by the CDC as a product that triggers immunity, while “vaccination” was described as an injection that prevents a disease, according to archived versions of the page. However, a flood of inquiries on the definitions was triggered by the fact that the COVID-19 vaccines have been increasingly ineffective against infection by the virus that causes COVID-19, the emails show.
“Our question is how is the CDC and the rest of the world allowed to call the shot a vaccination when it doesn’t even meet your own definition,” one person wrote to the CDC.
“Right-wing covid-19 pandemic deniers are using your ‘vaccine’ definition to argue that mRNA vaccines are not vaccines,” another said.
The Pfizer and Moderna COVID-19 vaccines are both built on messenger RNA technology. They are two of the three COVID-19 vaccines available in the United States.
Downs and colleagues Allison Michelle Fisher, Cynthia Jorgensen, Valerie Morelli, and Andrew (no last name given) worked on changing the definitions for “vaccine” and “vaccination,” according to the emails. [MORE]
A recently filed lawsuit funded by a renowned IP underwriter and analyst Dr. David Martin challenging the CMS federal mandate explains the importance of the definition vaccine and the legal distinction between vaccine and treatment because it triggers a much higher and stricter level of judicial review of mandates. The complaint in relevant part states "the CMS Mandate must be struck down because:
The overwhelming evidence shows that the Injections do not prevent transmission, infection, or reinfection in those who receive them.
The CDC Director has admitted that the Injections do not prevent infection or transmission of SARS-CoV-2, the virus that has been identified by various public health agencies as causing the disease known as COVID-19. “[W]hat [the vaccines] can’t do anymore is prevent transmission.”1
The CDC has acknowledged that the “vaccinated” and “unvaccinated” are equally likely to spread the virus.2 The Injections do not confer immunity but are claimed to reduce the severity of symptoms experienced by those infected by SARS-CoV-2. They are, therefore, treatments and not vaccines as that term has always been defined in the law.
In fact, the CDC has actually changed its definitions of “vaccine” and “vaccination” so that the Injections would fit within the new definition. Until recently, the Centers for Disease Control defined a “Vaccine” as: “A product that stimulates a person’s immune system to produce immunity to a specific disease, protecting the person from that disease.”3
The CDC also previously defined “Vaccination” as: “The act of introducing a vaccine into the body to produce immunity to a specific disease.”4
Both prior definitions fit the common understanding of those terms. To be vaccinated meant that the recipient should have lasting, robust immunity to the disease targeted by the vaccine.
But on September 1, 2021, the CDC quietly rewrote these definitions. It changed the definition of a “Vaccine” to: “A product that stimulates a person’s immune system to produce immunity to a specific disease, protecting the person from that disease preparation that is used to stimulate the body’s immune response against diseases.”5 It changed the definition of “Vaccination” to: “The act of introducing a vaccine into the body to produce immunity to protection from a specific disease.”6
Thus, the CDC has eliminated the word “immunity” from its definitions of “Vaccine” and “Vaccination.” Upon information and belief, the CDC did so because it recognizes that the Injections do not produce immunity to the disease known as COVID-19.
This is a critical factual and legal distinction. The Supreme Court has long held that the right to refuse medical treatment is a fundamental human right. Since the Injections do not stop the transmission of SARS-CoV-2 as a matter of fact, they are not “vaccines” as a matter of law. Instead, they are a therapeutic or medical treatment which Dr. Griner has the fundamental human right to refuse.
The complaint further explains,
The complaint explains,
“Because the Injections are treatments, and not vaccines, strict scrutiny applies. The US Supreme Court has recognized a “general liberty interest in refusing medical treatment.” Cruzan v. Dir., Mo. Dep’t of Health, 497 U.S. 261, 278, 110 S. Ct. 2841, 2851, 111 L.Ed.2d 224, 242 (1990). It has also recognized that the forcible injection of medication into a nonconsenting person’s body represents a substantial interference with that person’s liberty. Washington v. Harper, 494 U.S. 210, 229, 110 S. Ct. 1028, 1041, 108 L.Ed.2d 178, 203 (1990), see also id. at 223 (further acknowledging in dicta that, outside of the prison context, the right to refuse treatment would be a “fundamental right” subject to strict scrutiny).32
As mandated medical treatments are a substantial burden, Defendants must prove that the CMS Mandate is narrowly tailored to meet a compelling interest.
No such compelling interest exists because, as alleged above, the Injections are not effective against the now dominant Omicron variant of SARS-CoV-2 in that they do not prevent the recipient from becoming infected, getting reinfected, or transmitting SARS-CoV-2 to others. Indeed, evidence shows that vaccinated individuals have more SARS-CoV-2 in their nasal passages than unvaccinated people do.
The Injections may have been somewhat effective against the original SARS-CoV- 2 strain, but that strain has come and gone, and the Injections—designed to fight yesterday’s threat—are simply ineffective against the current variant.
Since the Injections are ineffective against the Delta and Omicron viral variants, and the original variant has been supplanted, there can be no compelling interest to mandate their use at this time.”
But even if there were a compelling interest in mandating the Injections, the CMS Mandate is not narrowly tailored to achieve such an interest.
The blanket mandate ignores individual factors increasing or decreasing the risks that the plaintiff—indeed, all healthcare workers—pose to themselves or to others.
Defendants entirely disregard whether employees have already obtained natural immunity despite the fact that natural immunity does actually provide immunity whereas the Injections do not.
Treating all employees the same, regardless of their individual medical status, risk factors, and natural immunity status is not narrowly tailored.
Moreover, the CMS Mandate fails entirely to consider other existing treatment options beyond the Injections as part of a more narrowly tailored approach. 97. Given these facts, as more fully set forth above, the CMS Mandate has no real or substantial relation to public health or is beyond all question, a plain, palpable invasion of rights secured by the fundamental law. Alternatively, the CMS Mandate has no real or substantial relation to public health or is beyond all question, a plain, palpable invasion of rights secured by the fundamental law as to Plaintiff, who already has natural immunity.” [MORE]
While Boris Johnson was "Resigning," a UK Government Report Revealed that 94% of all COVID Deaths in April and May 2022 were Among the Fully Vaccinated and 90% were Triple or Quadruple-Jabbed
/From [EXPOSE] and [MERCOLA] While eyes around the world were focusing on British Prime Minister Boris Johnson’s resignation, a report on COVID-19 cases and deaths was quietly issued in the U.K., and now it’s raising eyebrows.
According to the U.K. Office for National Statistics, 94% of all COVID deaths in April and May 2022 were among fully vaccinated individuals. Not only that, 90% were triple- or quadruple-jabbed.
“It’s times like these that you ought to watch what bad news is being quietly published in the background in the hope that it won’t receive much attention, and it just so happens that hours before Boris announced his resignation, his government published new data that proves things have been terrible for the vaccinated population in England over the past couple of months,” The Exposé said.
In total, there were 4,935 COVID deaths, and 4,647 were considered fully vaccinated. Of those deaths, 4,216 were triple-jabbed. On the flip side, only 288 deaths were recorded among those who had not received the shots.
The following chart shows the real world Covid-19 vaccine effectiveness among the triple vaccinated population in England in the Week 3, Week 7 and Week 13 UKHSA Vaccine Surveillance reports of 2022 –
This was nowhere near the claimed 95% effectiveness by Pfizer was it?
But now we have more evidence to both prove the UKHSA was lying, and that the current mainstream media storm surrounding the resignation of Boris Johnson is just a distraction.
This is because another UK Government agency, known as the Office for National Statistics (ONS), has just published data on deaths by vaccination status.
The latest dataset from the ONS is titled ‘Deaths by Vaccination Status, England, 1 January 2021 to 31 May 2022‘, and it can be accessed on the ONS site here, and downloaded here.
Table 1 of the latest dataet contains figures on the mortality rates by vaccination status for all cause deaths, deaths involving Covid-19, and deaths not involving Covid-19. And it is here that we are able to ascertain the vaccination status of everyone who has died of Covid-19 since the beginning of April 2022, when the UKHSA claimed they could no longer reliably report the figures.
Here’s how the ONS presents the figures for the month of April 2022 –
We’ve taken the figures provided by the ONS for both April and May 2022, and produced the following chart showing Covid-19 deaths by vaccination status in England between 1st April and 31st May 2022 –
In all, according to the ONS, there were 4,935 Covid-19 deaths over these two months, and the vaccinated population accounted for a shocking 4,647 of those deaths. But what’s even more shocking is that the triple vaccinated accounted for 4,216 of those deaths, with just 288 deaths recorded among the unvaccinated population.
In March 2022, there were 321 Covid-19 deaths within 60 days of a positive test among the unvaccinated population according to the UKHSA Week 13 Vaccine Surveillance Report, as detailed in the following chart using data extracted from table 13b of the report –
This means there were 33 less deaths among the unvaccinated over two months (April and May) than there were in the whole of March.
Unfortunately, the opposite is true for the vaccinated population, especially the triple vaccinated. There were 911 more deaths among the vaccinated over two months, and 1,161 more death among the triple vaccinated.
This means deaths have fallen drastically among the unvaccinated but increased significantly among the vaccinated population since the UKHSA claimed they could no longer reliably publish the data.
The following chart shows the percentage of Covid-19 deaths by vaccination status in England between 1st April and 31st May 2022, according to the latest ONS dataset published just hours before Boris Johnson’s resignation –
The quietly published figures reveal that the vaccinated population as a whole accounted for a shocking 94% of all Covid-19 deaths in April and May 2022, with the unvaccinated accounting for just 6% of all Covid-19 deaths. But the most horrific statistic here is that 90% of the deaths among the vaccinated were among people who had been given at least three doses of a Covid-19 injection.
However, many in the UK have been given a fourth dose of a Covid-19 injection since the spring, and judging by the latest data from the Government of Cananda, it’s likely many of those deaths could actually be among the quadruple vaccinated.
The most recent Government of Canada figures show that there were 521 Covid-19 deaths between 6th and 12th June, and the vaccinated population accounted for 485 of them, with a shocking 242 deaths among the quadruple vaccinated population, meaning they accounted for 50% of Covid-19 deaths among the vaccinated in the second week of June 2022.
These aren’t the kind of figures you would expect to see if the Covid-19 injections really are up to 95% effective at preventing death, are they? [MORE]
Canadian Gov Data Shows that in June 2022, the Vaccinated Accounted for 93% of all COVID Deaths; 50% of which were Quadruple Jabbed
/From [EXPOSE] The following chart shows the number of Covid-19 cases across the whole of Canada by vaccination status between 6th June and 12th June 2022 –
the most recent figures show that there were 17,904 Covid-19 cases between 6th and 12th June, and the vaccinated population accounted for 17,040 of them, with 13,147 cases among the quadruple vaccinated population.
This means the unvaccinated population accounted for 5% of Covid-19 cases, whilst the vaccinated population accounted for 95%, 77% of which were among the quadruple jabbed.
Covid-19 Hospitalisations
The following chart shows the number of Covid-19 hospitalisations across the whole of Canada by vaccination status between 6th June and 12th June 2022 –
The most recent figures show that there were 1,041 Covid-19 hospitalisations between 6th and 12th June, and the vaccinated population accounted for 938 of them, with 694 hospitalisations among the quadruple vaccinated population.
This means the unvaccinated population accounted for 10% of Covid-19 hospitalisations, whilst the vaccinated population accounted for 90%, 74% of which were among the quadruple jabbed.
Covid-19 Deaths
The following chart shows the number of Covid-19 deaths across the whole of Canada by vaccination status between 6th June and 12th June 2022 –
The most recent figures show that there were 521 Covid-19 deaths between 6th and 12th June, and the vaccinated population accounted for 485 of them, with a shocking 242 deaths among the quadruple vaccinated population, and 200 deaths among the triple vaccinated population.
This means the unvaccinated population accounted for just 7% of Covid-19 deaths, whilst the vaccinated population accounted for 93%, 50% of which were among the quadruple jabbed, and 41% of which were among the triple jabbed.
Despite a mass booster campaign, and the Government of Canada trying to desperately conceal it, a bit of time, effort, and simple maths has revealed that 9 in every 10 Covid-19 cases, hospitalisations and deaths were recorded among the fully vaccinated population between 6th and 12th June 2022, and the vast majority of those were among the quadruple jabbed.
Should we really be seeing this if the Covid-19 injections are effective?
Absolutely not. These figures suggest the more jabs you have, the more likely you are to be hospitalised or lose your life if exposed to the alleged Covid-19 virus.
FDA Quietly Grants Full Approval of Comirnaty Vax for Teens. Yet, It's not available in the US and Not the Same Formula as the Emergency Authorized Vax Falsely being Distributed as “fully approved”
/From [CHD] The U.S. Food and Drug Administration (FDA) on Friday granted full approval of Pfizer-BioNTech’s Comirnaty COVID-19 vaccine for adolescents 12 through 15 years old.
In an FDA press release, the agency said full approval of Comirnaty follows a “rigorous analysis and evaluation of the safety and effectiveness data,” and the Pfizer-BioNTech vaccine “has been, and will continue to be authorized for emergency use in this age group since May 2021.”
Pfizer’s press release announcing the approval said the Comirnaty vaccine has been available under Emergency Use Authorization (EUA) since May 2021 for the adolescent age group.
Yet, Comirnaty is not available in the U.S for any age group and is not the same formula as the Pfizer-BioNTech vaccine currently authorized under EUA and being distributed as a “fully approved” vaccine.
“The approval of Comirnaty for adolescents 12 to 15 is head-spinning,” said Mary Holland, president and general counsel for Children’s Health Defense.
Holland added:
“The FDA failed to convene an expert committee and failed to appropriately weigh the risk-benefit profile of this vaccine for this age group. Even Vaccine cheerleader Dr. Paul Offit acknowledged FDA decisions are being made based on political pressure, not science when, in commenting on the agency’s vote last week to allow reformulated booster shots, he said it felt like ‘the fix was in.’”
Holland said that at base, “this is a move by pharma to ensure liability protection” under the National Childhood Vaccine Injury Act of 1986. Some states likely will attempt to put Comirnaty on the childhood vaccine schedule, despite the myriad known and unknown risks, Holland said.
“Pfizer‘s fraud and collusion with government is becoming more evident by the day,” Holland said. “CHD, already challenging the authorizations for those six months through age 11, will be at the forefront of challenging this approval for teenagers.”
Efficacy claims based on old analysis of 16- to 25-year-olds — before Delta, Omicron variants
Pfizer said Friday’s approval is based on data from a Phase 3 clinical trial of 2,260 participants ages 12 through 15.
About half of the participants, “elicited SARS-CoV-2–neutralizing antibody geometric mean titers (GMTs)” demonstrating “strong immunogenicity in a subset of adolescents one month after the second dose,” Pfizer said.
It is unknown what happened to antibody levels after one month, but peer-reviewed researchsuggests vaccine protection conferred by second and third doses of Pfizer’s COVID-19 vaccine wanes rapidly against the Omicron variant.
“Our study found a rapid decline in Omicron-specific serum neutralizing antibody titers only a few weeks after the second and third doses of [the Pfizer-BioNTech] BNT162b2,” said the authors of a May 13 study published in JAMA.
BAIT AND SWITCH WITH THE DEPENDENT MEDIA’S HELP: CONFUSE TO DESTROY INFORMED CONSENT. LAST MONTH PFIZER ADMITTED IT WILL NEVER MANUFACTURE THE VACCINE THAT WAS FDA APPROVED. PFIZER QUIETLY SUBMITTED AN UPDATE TO THE CDC, ADMITTING THAT ITS ORIGINALLY LICENSED “COMIRNATY” VACCINE WILL NEVER BE DISTRIBUTED FOR USE. PFIZER HAS EXCLUSIVELY BEEN SUPPLYING ITS VERSION OF THE EXPERIMENTAL VACCINE THAT WAS GRANTED EMERGENCY USE AUTHORIZATION (EUA) BY THE FDA. [MORE]
To further support its claim that Comirnaty is effective in the 12 to 15 age group, Pfizer used an old analysis of 16- to 25-year-olds conducted before the Delta and Omicron surges.
“The efficacy analysis was conducted between November 2020 and May 2021, which was before the Delta and Omicron surges,” and the “only SARS-CoV-2 variant of concern identified from the confirmed COVID-19 cases in this age group was Alpha,” Pfizer said in its press release.
FDA experts question neutralizing antibodies as standard for vaccine effectiveness
During a June 28 meeting of the FDA’s Vaccine and Related Biological Products Advisory Committee (VRBPAC), vaccine experts raised concerns that neutralizing antibodies did not correlate to clinical protection — noting Moderna’s COVID-19 vaccine had a two-fold increase in neutralizing antibody levels compared with Pfizer’s vaccine during clinical trials, but it did not translate into a clinically significant difference in terms of protection against severe disease.
Dr. Ofer Levy, VRBPAC member and infectious disease physician at Boston Children’s Hospital, said during the meeting there is still “no established correlate of protection,” referring to the level of antibodies needed to confer protection.
“You have a lot of data now,” Levy told Pfizer. “What is your relative protection?”
“I would say there is no established correlate of protection,” Kena Swanson, Ph.D., vice president of viral vaccines at Pfizer, told Levy.
Levy said:
“I would like to hear from FDA what their overall approach will be around improving our understanding of correlate protection. We spend a good amount of time reviewing antibody data. We have no doubt antibody data is important. We don’t have a level of antibody that anybody is comfortable stating is correlated [with] protection.
Levy, who said antibodies are important, but T cells are more important, called for federal leadership to establish a “standardization of the T-cell assay and encourage or in fact require the sponsors to gather that information.”
“So what is the effort to standardize the pre-clinical assays?” Levy asked. “This is an effort that’s critical not just now but for future cycles of vaccine revision. If we aren’t able to define a standard for correlate protection we are fighting with one arm behind our back.”
Dr. Peter Marks, head of the FDA’s Center for Biologics Evaluation and Research, acknowledged the importance of Levy’s question and said they are “having conversations” with colleagues at the National Institutes of Health and throughout government about how they might move forward, but it is something they “don’t have an answer to yet.”
Marks said as vaccines are developed in the future, it will “become even more important” to define a standard of correlate protection because “we won’t be able to have a large naive population to vaccinate with newer vaccines.”
“We will need to understand the T-cell response better,” Marks said. “I take your point, it’s just that we haven’t solved the problem yet.“
Comirnaty not available in the U.S.
According to Pfizer’s press release, Comirnaty was previously made available to the 12 to 15 age group in the U.S. under EUA and 9 million U.S. adolescents in this age group have completed a primary series.
“The vaccine, sold under the brand name Comirnaty for adults, has been available under an emergency use authorization since May 2021 for the 12-15 age group,” Reuters reported. “It will now be sold under the same brand name for adolescents as well.”
Yet, Pfizer’s information hotline says it has no specific information on when Comirnaty will be available.
The FDA said Friday the Pfizer-BioNTech vaccine “has been, and will continue to be, authorized for emergency use in this age group since May 2021.”
The CDC’s website states that Comirnaty is “not orderable.”
A branch of the U.S. Department of Health and Human Services overseeing the Strategic National Stockpile indicated Comirnaty was not available because Pfizer did not have time to change the labels.
According to FDA documents, Comirnaty is not available in the U.S. and nobody has received a fully approved and licensed COVID-19 vaccine.
“Comirnaty has not been made available under EUA,” said Dr. Madhava Setty, physician and senior science editor for The Defender.
Setty added:
“The FDA and Pfizer have already stated very quietly, that they have no intent of manufacturing Comirnaty for distribution. Everyone is getting the non-licensed formulation that carries no liability for pharmaceutical companies.”
The CDC website confirms this, stating the Comirnaty formulation “will not be manufactured or made available in the near term even if authorized.”
The FDA on Aug. 23, 2021, approved Pfizer’s biological licensing application (BLA) for its COVID-19 vaccine named Comirnaty for people age 16 and older.
CHD challenged FDA on Comirnaty ‘approval’ for adults
As The Defender reported, there were “several bizarre aspects to the FDA approval” that proved confusing — which led to CHD suing the FDA over its approval of Comirnaty.
The FDA acknowledged that while Pfizer had “insufficient stocks” of the newly licensed Comirnaty vaccine, there was “a significant amount” of the Pfizer-BioNTech COVID vaccine — produced under EUA — still available for use.
The FDA said the Pfizer-BioNTech vaccine under EUA should remain unlicensed but could be used “interchangeably” with the newly licensed Comirnaty product.
The FDA also said the licensed Pfizer Comirnaty vaccine and the existing Pfizer-BioNTech vaccine were “legally distinct,” but proclaimed their differences did not “impact safety or effectiveness.”
Yet, there is a “huge real-world difference” between products approved under EUA compared with those the FDA has fully licensed.
EUA products are experimental under U.S. law and cannot be mandated. A licensed vaccine, such as Comirnaty, can be mandated by employers and schools.
Although Pfizer’s Comirnaty vaccine can be mandated, it has no liability shield. Vials of the branded product, which say “Comirnaty” on the label, are subject to the same product liability laws as other U.S. products.
Only COVID-19 vaccines distributed under EUA — which in the U.S. includes Pfizer-BioNTech, Moderna and Johnson & Johnson — have liability protection under the 2005 Public Readiness and Preparedness Act (PREP).
Under PREP, the only way an injured party can sue a pharmaceutical company for an injury caused by an EUA vaccine is if he or she can prove willful misconduct and if the U.S. government has also brought an enforcement action against the party for willful misconduct. No such lawsuit has ever succeeded.
Comirnaty cannot receive liability protection unless it is fully approved for children and added to the CDC’s immunization schedule bringing it under the auspices of the National Vaccine Injury Compensation Program.
Pfizer-BioNTech and Comirnaty vaccines aren’t interchangeable
The FDA on Oct. 29, 2021, authorized a manufacturing change to allow an additional formulation of the Pfizer-BioNTech COVID-19 vaccine that uses tromethamine (Tris) buffer instead of phosphate-buffered saline (PBS) used in the originally authorized Pfizer-BioNTech COVID-19 vaccine.
The FDA on Dec. 16, 2021, approved a supplement to the Comirnaty BLA to include a new 30 mcg dose formulation that uses the Tris buffer instead of the PBS buffer used in the originally approved vaccine.
The Pfizer-BioNTech vaccine may contain either the PBS buffer or tris buffer, except for the 5 to 11 age group. The Comirnaty vaccine contains the Tris buffer.
The Pfizer-BioNTech vaccine used for the 5 to 11 age group uses a Tris buffer, despite clinical trialshaving been conducted using Pfizer’s vaccine containing the PBS buffer.
According to Pfizer’s July 8 press release, the FDA relied upon studies conducted prior to the formula change to justify the approval of Pfizer’s Comirnaty vaccine for adolescents ages 12 to 15.
The type of buffer used in a COVID-19 vaccine can affect the potency of the vaccine, how it is stored and the propensity to develop potential adverse events, TrialSite News reported.
According to Cleveland Clinic, Tris is commonly used for the prevention and treatment of metabolic acidosis associated with various clinical conditions such as heart bypass surgery or cardiac arrest. It is also used in other vaccines, including Moderna’s COVID-19 vaccine, dengue, smallpox and Ebola vaccines.
The FDA categorizes tromethamine as a category C drug and suggests using tromethamine only if clearly needed.
It is unknown if tromethamine will harm an unborn baby, but animal reproduction studies have shown an adverse effect on the fetus, and there are “no adequate and well-controlled studies in humans.”
“The FDA-evaluated manufacturing data [to] support the change in this inactive ingredient and concluded it did not impact the safety or effectiveness of the product,” Marks, said during an October 2021, press briefing.
According to the FDA’s Letter of Authorization, reissued on Oct. 29, “analytical comparability assessments” revealed the Pfizer-BioNTech COVID vaccine formulations containing Tris and PBS buffers were “analytically comparable.”
Yet, no human or animal trials were conducted to determine the safety or efficacy of the new formula.